LIMSwiki
Contents
| |
| Names | |
|---|---|
| IUPAC name
Nitrous acid[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.029.057 |
| EC Number |
|
| 983 | |
| KEGG | |
| MeSH | Nitrous+acid |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
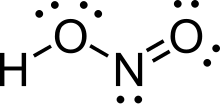
| HNO2 | |
| Molar mass | 47.013 g/mol |
| Appearance | Pale blue solution |
| Density | Approx. 1 g/ml |
| Melting point | Only known in solution or as gas |
| Acidity (pKa) | 3.15[2] |
| Conjugate base | Nitrite |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Nitric acid |
Other cations
|
Sodium nitrite Potassium nitrite Ammonium nitrite |
Related compounds
|
Dinitrogen trioxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Nitrous acid (molecular formula HNO
2) is a weak and monoprotic acid known only in solution, in the gas phase, and in the form of nitrite (NO−
2) salts.[3] It was discovered by Carl Wilhelm Scheele, who called it "phlogisticated acid of niter". Nitrous acid is used to make diazonium salts from amines. The resulting diazonium salts are reagents in azo coupling reactions to give azo dyes.
Structure
In the gas phase, the planar nitrous acid molecule can adopt both a syn and an anti form. The anti form predominates at room temperature, and IR measurements indicate it is more stable by around 2.3 kJ/mol.[3]
-
Dimensions of the anti form
(from the microwave spectrum) -
Model of the anti form
-
syn form
Preparation and decomposition
Free, gaseous nitrous acid is unstable, rapidly disproportionating to nitric oxides:
- 2 HNO2 → NO2 + NO + H2O
In aqueous solution, the nitrogen dioxide also disproportionates, for a net reaction producing nitric oxide and nitric acid:[4]: 1 [5]
- 3 HNO2 → 2 NO + HNO3 + H2O
Consequently applications of nitrous acid usually begin with mineral acid acidification of sodium nitrite. The acidification is usually conducted at ice temperatures, and the HNO2 consumed in situ.[6][7]
Nitrous acid equilibrates with dinitrogen trioxide in water, so that concentrated solutions are visibly blue:[4]: 2
- N2O3 + H2O ⇌ 2 HNO2
Addition of dinitrogen trioxide to water is thus another preparatory technique.
Chemical applications
Nitrous acid is the main chemophore in the Liebermann reagent, used to spot-test for alkaloids.
At high acidities (pH ≪ 2), nitrous acid is protonated to give water and nitrosonium cations.[4]: 2
Reduction
With I− and Fe2+ ions, NO is formed:[8]
- 2 HNO2 + 2 KI + 2 H2SO4 → I2 + 2 NO + 2 H2O + 2 K2SO4
- 2 HNO2 + 2 FeSO4 + 2 H2SO4 → Fe2(SO4)3 + 2 NO + 2 H2O + K2SO4
With Sn2+ ions, N2O is formed:
- 2 HNO2 + 6 HCl + 2 SnCl2 → 2 SnCl4 + N2O + 3 H2O + 2 KCl
With SO2 gas, NH2OH is formed:
- 2 HNO2 + 6 H2O + 4 SO2 → 3 H2SO4 + K2SO4 + 2 NH2OH
With Zn in alkali solution, NH3 is formed:
- 5 H2O + KNO2 + 3 Zn → NH3 + KOH + 3 Zn(OH)2
With N
2H+
5, both HN3 and (subsequently) N2 gas are formed:
- HNO2 + [N2H5]+ → HN3 + H2O + H3O+
- HNO2 + HN3 → N2O + N2 + H2O
Oxidation by nitrous acid has a kinetic control over thermodynamic control, this is best illustrated that dilute nitrous acid is able to oxidize I− to I2, but dilute nitric acid cannot.
- I2 + 2 e− ⇌ 2 I− Eo = +0.54 V
- NO−
3 + 3 H+ + 2 e− ⇌ HNO2 + H2O Eo = +0.93 V
- HNO2 + H+ + e− ⇌ NO + H2O Eo = +0.98 V
It can be seen that the values of Eo
cell for these reactions are similar, but nitric acid is a more powerful oxidizing agent. Base on the fact that dilute nitrous acid can oxidize iodide into iodine, it can be deduced that nitrous is a faster, rather than a more powerful, oxidizing agent than dilute nitric acid.[8]
Organic chemistry
Nitrous acid is used to prepare diazonium salts:
- HNO2 + ArNH2 + H+ → ArN+
2 + 2 H2O
where Ar is an aryl group.
Such salts are widely used in organic synthesis, e.g., for the Sandmeyer reaction and in the preparation azo dyes, brightly colored compounds that are the basis of a qualitative test for anilines.[9] Nitrous acid is used to destroy toxic and potentially explosive sodium azide. For most purposes, nitrous acid is usually formed in situ by the action of mineral acid on sodium nitrite:[10] It is mainly blue in colour
- NaNO2 + HCl → HNO2 + NaCl
- 2 NaN3 + 2 HNO2 → 3 N2 + 2 NO + 2 NaOH
Reaction with two α-hydrogen atoms in ketones creates oximes, which may be further oxidized to a carboxylic acid, or reduced to form amines. This process is used in the commercial production of adipic acid.
Nitrous acid reacts rapidly with aliphatic alcohols to produce alkyl nitrites, which are potent vasodilators:
- (CH3)2CHCH2CH2OH + HNO2 → (CH3)2CHCH2CH2ONO + H2O
The carcinogens called nitrosamines are produced, usually not intentionally, by the reaction of nitrous acid with secondary amines:
- HNO2 + R2NH → R2N-NO + H2O
Atmosphere of the Earth
Nitrous acid is involved in the ozone budget of the lower atmosphere, the troposphere. The heterogeneous reaction of nitric oxide (NO) and water produces nitrous acid. When this reaction takes place on the surface of atmospheric aerosols, the product readily photolyses to hydroxyl radicals.[11][12]
DNA damage and mutation
Treatment of Escherichia coli cells with nitrous acid causes damage to the cell's DNA including deamination of cytosine to uracil, and these damages are subject to repair by specific enzymes.[13] Also, nitrous acid causes base substitution mutations in organisms with double-stranded DNA.[14]
See also
References
- ^ "Nitrous Acid".
- ^ Perrin, D. D., ed. (1982) [1969]. Ionisation Constants of Inorganic Acids and Bases in Aqueous Solution. IUPAC Chemical Data (2nd ed.). Oxford: Pergamon (published 1984). Entry 156. ISBN 0-08-029214-3. LCCN 82-16524.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8. p. 462.
- ^ a b c Williams, D. L. H. (1988). Nitrosation. Cambridge, UK: Cambridge University. ISBN 0-521-26796-X.
- ^ Kameoka, Yohji; Pigford, Robert (February 1977). "Absorption of Nitrogen Dioxide into Water, Sulfuric Acid, Sodium Hydroxide, and Alkaline Sodium Sulfite Aqueous". Ind. Eng. Chem. Fundamen. 16 (1): 163–169. doi:10.1021/i160061a031.
- ^ Petit, Y.; Larchevêque, M. (1998). "Ethyl Glycidate from (S)-Serine: Ethyl (R)-(+)-2,3-Epoxypropanoate". Org. Synth. 75: 37. doi:10.15227/orgsyn.075.0037.
- ^ Smith, Adam P.; Savage, Scott A.; Love, J. Christopher; Fraser, Cassandra L. (2002). "Synthesis of 4-, 5-, and 6-methyl-2,2'-bipyridine by a Negishi Cross-coupling Strategy: 5-methyl-2,2'-bipyridine". Org. Synth. 78: 51. doi:10.15227/orgsyn.078.0051.
- ^ a b Housecroft, Catherine E.; Sharpe, Alan G. (2008). "Chapter 15: The group 15 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 449. ISBN 978-0-13-175553-6.
- ^ Clarke, H. T.; Kirner, W. R. (1922). "Methyl Red". Organic Syntheses. 2: 47. doi:10.15227/orgsyn.002.0047.
- ^ Prudent practices in the laboratory: handling and disposal of chemicals. Washington, D.C.: National Academy Press. 1995. doi:10.17226/4911. ISBN 978-0-309-05229-0.
- ^ Spataro, F; Ianniello, A (November 2014). "Sources of atmospheric nitrous acid: state of the science, current research needs, and future prospects". Journal of the Air & Waste Management Association. 64 (11): 1232–1250. Bibcode:2014JAWMA..64.1232S. doi:10.1080/10962247.2014.952846. PMID 25509545.
- ^ Anglada, Josef M.; Solé, Albert (November 2017). "The Atmospheric Oxidation of HONO by OH, Cl, and ClO Radicals". The Journal of Physical Chemistry A. 121 (51): 9698–9707. Bibcode:2017JPCA..121.9698A. doi:10.1021/acs.jpca.7b10715. PMID 29182863.
- ^ Da Roza, R.; Friedberg, E. C.; Duncan, B. K.; Warner, H. R. (1977-11-01). "Repair of nitrous acid damage to DNA in Escherichia coli". Biochemistry. 16 (22): 4934–4939. doi:10.1021/bi00641a030. ISSN 0006-2960. PMID 334252.
- ^ Hartman, Z.; Henrikson, E. N.; Hartman, P. E.; Cebula, T. A. (1994). "Molecular models that may account for nitrous acid mutagenesis in organisms containing double-stranded DNA". Environmental and Molecular Mutagenesis. 24 (3): 168–175. Bibcode:1994EnvMM..24..168H. doi:10.1002/em.2850240305. ISSN 0893-6692. PMID 7957120.





















