LIMSwiki
Contents
 | |
| Clinical data | |
|---|---|
| Other names | 17α-MP; 17α-Methylpregn-4-ene-3,20-dione |
| Drug class | Progestin; Progestogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H32O2 |
| Molar mass | 328.496 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
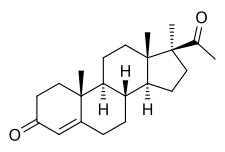
17α-Methylprogesterone (17α-MP), or 17α-methylpregn-4-ene-3,20-dione, is a steroidal progestin related to progesterone that was synthesized and characterized in 1949 but was never marketed.[1] Along with ethisterone (1938) and 19-norprogesterone (1951), 17α-MP was one of the earliest derivatives of progesterone to be identified as possessing progestogenic activity.[2] Similarly to progesterone and derivatives like 17α-hydroxyprogesterone and 19-norprogesterone, 17α-MP was found to possess poor (though not negligible) oral bioavailability,[3] but showed improved progestogenic activity relative to progesterone when administered via other routes (e.g., subcutaneous or vaginal).[4][5][6] In addition to its activity as a progestogen, 17α-MP has also been found to possess some antiglucocorticoid activity.[7]
The observation of two-fold improved potency of 17α-MP relative to progesterone led to renewed interest in 17α-substituted derivatives of progesterone.[5] Subsequently, hydroxyprogesterone acetate and hydroxyprogesterone caproate were synthesized in 1953 and introduced in 1956 and 1957, respectively, and medroxyprogesterone acetate was discovered in 1957 and introduced in 1959.[5] In addition, though 17α-MP itself was never introduced for medical use, progestogen derivatives of the compound, including medrogestone (1966) and the 19-norprogesterone derivatives demegestone (1974), promegestone (1983), and trimegestone (2001), have been marketed.[8]
Chemistry
See also
References
- ^ Plattner PA, Heusser H, Herzig PT (February 1949). "Über Steroide und Sexualhormone. 159. Mitteilung. Die Synthese von 17-Methyl-progesteron". Helvetica Chimica Acta. 32 (1): 270–275. doi:10.1002/hlca.19490320138. PMID 18115956.
- ^ Zderic JA (1963). "Progestational hormones". In Florkin M, Stotz ED (eds.). Comprehensive Biochemistry. Vol. 10. Amsterdam: Elsevier. pp. 166–196. Archived from the original on 9 October 2016.
- ^ Martini L, Pecile A (1965). Hormonal steroids: biochemistry, pharmacology and therapeutics; proceedings. Academic Press. p. 95. ISBN 9780124753020.
Progesterone is devoid of appreciable oral activity, but, particularly at positions 6 and 17, substituents of metabolic importance are known to impart oral activity to the molecule (Fieser and Fieser, 1959). We wish to report the preparation of 6- and 21 -substituted 17- methylprogesterones, a class of substances which we synthesized after the observation that 17-methylprogesterone (Plattner et al., 1949), contrary to previous reports (Engel, 1951, 1960), was not devoid of oral activity (cf. Table I).
- ^ Shipley EG (3 February 2016). "Anti-Gonadotropic Steroids, Inhibition of Ovulation and Mating". In Dorfman RI (ed.). Bioassay. Elsevier. pp. 260–. ISBN 978-1-4832-7276-4.
17-Methylprogesterone is effective subcutaneously or intravaginally, but is ineffective orally.
- ^ a b c Applezweig N (1962). Steroid Drugs. Blakiston Division, McGraw-Hill. pp. 101–102.
In 1950, 17a-methyl-progesterone was shown to have twice the activity of progesterone. This led to a renewed interest in 17-substituted derivatives. [...] Junkmann of Schering, AG., however, was able to show that long chain esters of 17a-hydroxyprogesterones such as the 17a-caproate produced powerful long-acting progestational effect. This compound is marketed in the United States as Delalutin by Squibb, and has been heavily used for the treatment of habitual abortion. Subsequently, a series of events led to the exploitation of 17a-hydroxyprogesterone derivatives as highly effective and orally active progestogens. Groups at Upjohn, Merck & Co., and Syntex independently found means of readily acetylating the 17-hydroxy group. Later, Upjohn announced it found that 17a-acetoxyprogesterone was orally active in humans and subsequently marketed this compound under the name of Prodox. The idea that progesterone could be protected by substitution against metabolic destruction was not exclusive to one group of investigators. [...] Upjohn later brought out the 6a-methyl-17a-acetoxyprogesterone under the name of Provera. This is ten to twenty-five times as active as ethisterone by oral route.
- ^ Tullner WW, Hertz R (March 1953). "High progestational activity of 19-norprogesterone". Endocrinology. 52 (3): 359–361. doi:10.1210/endo-52-3-359. PMID 13033848.
17-methylprogesterone A has twice the activity of progesterone using the Corner-Allen assay method (Heuser et al., 1950).
- ^ Duncan MR, Duncan GR (March 1979). "An in vivo study of the action of antiglucocorticoids on thymus weight ratio, antibody titre and the adrenal-pituitary-hypothalamus axis". Journal of Steroid Biochemistry. 10 (3): 245–259. doi:10.1016/0022-4731(79)90250-4. PMID 222960.
- ^ Revesz C, Chappel CI (December 1966). "Biological activity of medrogestone: a new orally active progestin". Journal of Reproduction and Fertility. 12 (3): 473–487. doi:10.1530/jrf.0.0120473. PMID 4288903.
The desirability of an orally active progestin which would be free from these objectionable properties led to the synthesis of a series of derivatives of 17-methylprogesterone (Deghenghi & Gaudry, 1961; Deghenghi, Revesz & Gaudry, 1963) and their testing as progestational agents. The biological activity of 6-methyl-6-dehydro-17-methylprogesterone, which appeared to be the most promising member of this series, [...]

















