Trends in LIMS
Contents
| S100/ICaBP type calcium binding domain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
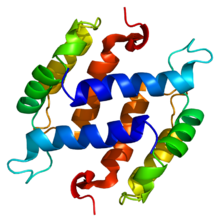 Structure of the S100B protein. Based on PyMOL rendering of PDB 1b4c. | |||||||||||
| Identifiers | |||||||||||
| Symbol | S_100 | ||||||||||
| Pfam | PF01023 | ||||||||||
| InterPro | IPR013787 | ||||||||||
| PROSITE | PDOC00275 | ||||||||||
| SCOP2 | 1cnp / SCOPe / SUPFAM | ||||||||||
| |||||||||||
The S100 proteins are a family of low molecular-weight proteins found in vertebrates characterized by two calcium-binding sites that have helix-loop-helix ("EF-hand-type") conformation. At least 21 different S100 proteins are known.[1] They are encoded by a family of genes whose symbols use the S100 prefix, for example, S100A1, S100A2, S100A3. They are also considered as damage-associated molecular pattern molecules (DAMPs), and knockdown of aryl hydrocarbon receptor downregulates the expression of S100 proteins in THP-1 cells.[2]
Structure
Most S100 proteins consist of two identical polypeptides (homodimeric), which are held together by noncovalent bonds. They are structurally similar to calmodulin. They differ from calmodulin, though, on the other features. For instance, their expression pattern is cell-specific, i.e. they are expressed in particular cell types. Their expression depends on environmental factors. In contrast, calmodulin is a ubiquitous and universal intracellular Ca2+ receptor widely expressed in many cells.
Normal function
S100 proteins are normally present in cells derived from the neural crest (Schwann cells, and melanocytes), chondrocytes, adipocytes, myoepithelial cells, macrophages, Langerhans cells,[3][4] dendritic cells,[5] and keratinocytes. They may be present in some breast epithelial cells.
S100 proteins have been implicated in a variety of intracellular and extracellular functions,[6] such as regulation of protein phosphorylation, transcription factors, Ca2+ homeostasis, the dynamics of cytoskeleton constituents, enzyme activities, cell growth and differentiation, and the inflammatory response. S100A7 (psoriasin) and S100A15 have been found to act as cytokines in inflammation, particularly in autoimmune skin conditions such as psoriasis.[7]
Pathology
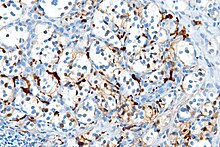
Several members of the S100 protein family are useful as markers for certain tumors and epidermal differentiation. They can be found in melanomas,[8] 100% of schwannomas, 100% of neurofibromas (weaker than schwannomas), 50% of malignant peripheral nerve sheath tumors (may be weak and/or focal), paraganglioma stromal cells, histiocytoma, and clear-cell sarcomas. Further, S100 proteins are markers for inflammatory diseases and can mediate inflammation and act as antimicrobials.[9] S100 proteins have been used in the lab as cell markers for anatomic pathology.
Human genes
- S100A1, S100A2, S100A3, S100A4, S100A5, S100A6, S100A7 (psoriasin), S100A7A (koebnerisin), S100A8 (calgranulin A), S100A9 (calgranulin B), S100A10, S100A11, S100A12 (calgranulin C), S100A13, S100A14, S100A16
- S100B, S100G, S100P,[10][11] S100Z (S100Z)
- CRNN, FLG, FLG2, HRNR, RPTN, TCHH, THHL1
Nomenclature
The "S100" symbol prefix denotes that these proteins are soluble in 100%, i.e. saturated, ammonium sulfate at neutral pH. The symbol has often been hyphenated,[12] but current gene and protein nomenclature, such as HUGO Gene Nomenclature Committee nomenclature, does not use hyphens in symbols.
See also
References
- ^ Marenholz I, Heizmann CW, Fritz G (2004). "S100 proteins in mouse and man: from evolution to function and pathology (including an update of the nomenclature)". Biochemical and Biophysical Research Communications. 322 (4): 1111–22. doi:10.1016/j.bbrc.2004.07.096. PMID 15336958.
- ^ Memari B, Bouttier M, Dimitrov V, Ouellette M, Behr MA, Fritz JH, White JH (2015). "Engagement of the Aryl Hydrocarbon Receptor in Mycobacterium tuberculosis-Infected Macrophages Has Pleiotropic Effects on Innate Immune Signaling". Journal of Immunology. 195 (9): 4479–91. doi:10.4049/jimmunol.1501141. PMID 26416282.
- ^ Wilson, AJ; Maddox, PH; Jenkins, D (January 1991). "CD1a and S100 antigen expression in skin Langerhans cells in patients with breast cancer". The Journal of Pathology. 163 (1): 25–30. doi:10.1002/path.1711630106. PMID 2002421. S2CID 70911084.
- ^ Coppola D, Fu L, Nicosia SV, Kounelis S, Jones M (1998). "Prognostic significance of p53, bcl-2, vimentin, and S100 protein-positive Langerhans cells in endometrial carcinoma". Human Pathology. 29 (5): 455–62. doi:10.1016/s0046-8177(98)90060-0. PMID 9596268.
- ^ Shinzato M, Shamoto M, Hosokawa S, Kaneko C, Osada A, Shimizu M, Yoshida A (1995). "Differentiation of Langerhans cells from interdigitating cells using CD1a and S-100 protein antibodies". Biotechnic & Histochemistry. 70 (3): 114–8. doi:10.3109/10520299509108327. PMID 7548432.
- ^ Donato R (2003). "Intracellular and extracellular roles of S100 proteins". Microscopy Research and Technique. 60 (6): 540–51. doi:10.1002/jemt.10296. PMID 12645002. S2CID 37798037.
- ^ Wolf R, Howard OM, Dong HF, Voscopoulos C, Boeshans K, Winston J, Divi R, Gunsior M, Goldsmith P, Ahvazi B, Chavakis T, Oppenheim JJ, Yuspa SH (2008). "Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15". Journal of Immunology. 181 (2): 1499–506. doi:10.4049/jimmunol.181.2.1499. PMC 2435511. PMID 18606705.
- ^ Nonaka D, Chiriboga L, Rubin BP (2008). "Differential expression of S100 protein subtypes in malignant melanoma, and benign and malignant peripheral nerve sheath tumors". Journal of Cutaneous Pathology. 35 (11): 1014–9. doi:10.1111/j.1600-0560.2007.00953.x. PMID 18547346. S2CID 22907221.
- ^ Wolf R, Ruzicka T, Yuspa SH (July 2010). "Novel S100A7 (psoriasin)/S100A15 (koebnerisin) subfamily: highly homologous but distinct in regulation and function". Amino Acids. 41 (4): 789–96. doi:10.1007/s00726-010-0666-4. PMC 6410564. PMID 20596736.
- ^ Penumutchu, Srinivasa R.; Chou, Ruey-Hwang; Yu, Chin (2014-08-01). "Structural Insights into Calcium-Bound S100P and the V Domain of the RAGE Complex". PLOS ONE. 9 (8): e103947. Bibcode:2014PLoSO...9j3947P. doi:10.1371/journal.pone.0103947. ISSN 1932-6203. PMC 4118983. PMID 25084534.
- ^ Penumutchu, Srinivasa R.; Chou, Ruey-Hwang; Yu, Chin (2014-10-17). "Interaction between S100P and the anti-allergy drug cromolyn". Biochemical and Biophysical Research Communications. 454 (3): 404–409. doi:10.1016/j.bbrc.2014.10.048. ISSN 1090-2104. PMID 25450399.
- ^ Elsevier, Dorland's Illustrated Medical Dictionary, Elsevier.
Further reading
- Wolf R, Voscopoulos CJ, FitzGerald PC, Goldsmith P, Cataisson C, Gunsior M, Walz M, Ruzicka T, Yuspa SH (2006). "The mouse S100A15 ortholog parallels genomic organization, structure, gene expression, and protein-processing pattern of the human S100A7/A15 subfamily during epidermal maturation". The Journal of Investigative Dermatology. 126 (7): 1600–8. doi:10.1038/sj.jid.5700210. PMID 16528363.
- Wolf R, Howard OM, Dong HF, Voscopoulos C, Boeshans K, Winston J, Divi R, Gunsior M, Goldsmith P, Ahvazi B, Chavakis T, Oppenheim JJ, Yuspa SH (2008). "Chemotactic activity of S100A7 (Psoriasin) is mediated by the receptor for advanced glycation end products and potentiates inflammation with highly homologous but functionally distinct S100A15". Journal of Immunology. 181 (2): 1499–506. doi:10.4049/jimmunol.181.2.1499. PMC 2435511. PMID 18606705.
- Wolf R, Voscopoulos C, Winston J, Dharamsi A, Goldsmith P, Gunsior M, Vonderhaar BK, Olson M, Watson PH, Yuspa SH (2009). "Highly homologous hS100A15 and hS100A7 proteins are distinctly expressed in normal breast tissue and breast cancer". Cancer Letters. 277 (1): 101–7. doi:10.1016/j.canlet.2008.11.032. PMC 2680177. PMID 19136201.
- Wolf R, Mascia F, Dharamsi A, Howard OM, Cataisson C, Bliskovski V, Winston J, Feigenbaum L, Lichti U, Ruzicka T, Chavakis T, Yuspa SH (2010). "Gene from a psoriasis susceptibility locus primes the skin for inflammation". Science Translational Medicine. 2 (61): 61ra90. doi:10.1126/scitranslmed.3001108. PMC 6334290. PMID 21148126.
External links
- S100+Proteins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)

















