Trends in LIMS
Contents
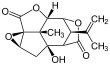
Picrotoxinin (left) and picrotin (right) | |||
| Clinical data | |||
|---|---|---|---|
| ATC code |
| ||
| Identifiers | |||
| CAS Number | |||
| PubChem CID | |||
| IUPHAR/BPS | |||
| DrugBank | |||
| ChemSpider | |||
| UNII | |||
| KEGG | |||
| ChEBI | |||
| ChEMBL | |||
| CompTox Dashboard (EPA) | |||
| ECHA InfoCard | 100.004.288 | ||
| Chemical and physical data | |||
| 3D model (JSmol) | |||
| |||
| |||
| | |||
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812.[1] The name "picrotoxin" is a combination of the Greek words "picros" (bitter) and "toxicon" (poison).[2] A mixture of two different compounds, picrotoxin occurs naturally in the fruit of the Anamirta cocculus plant, although it can also be synthesized chemically.
Due to its interactions with the inhibitory neurotransmitter GABA, picrotoxin acts as a stimulant and convulsant. It mainly impacts the central nervous system, causing seizures and respiratory paralysis in high enough doses.
Chemical structure and synthesis
Picrotoxin is an equimolar mixture of two compounds, picrotoxinin (C15H16O6; CAS# 17617-45-7) and picrotin (C15H18O7; CAS# 21416-53-5).[3] Of the two compounds, picrotin is less active.[4]
Picrotoxin occurs naturally in the fruit of the Anamirta cocculus, a climbing plant from India and other parts of Southeast Asia. The plant is known for its large stems of white wood and sweetly-scented flowers. It produces small stone fruits, Cocculus indicus, which are typically dried.[citation needed]
Currently, there are as many as five total syntheses of picrotoxinin — one of which was published as recently as June 2020.[5] Most syntheses use carvone as a stereochemical template.
![Begin with methyl (1S,4S,5R,7R,8S,9R,10R,11R)-10-(acetyloxy)-7-hydroxy-11-methyl-3-oxo-9-(prop-1-en-2-yl)-4,5-bis[(trimethylsilyl)oxy]-2-oxatricyclo[5.3.1.04,11]undecane-8-carboxylate. (1) Intramolecular transesterification, releasing methyl acetate; then (2) deprotection of a trimethylsilyl-protected vicinal diol, followed by (3) reductive dehydration to an olefin, and (4) stereospecific epoxidation to a glycidic ester](https://upload.wikimedia.org/wikipedia/commons/thumb/9/96/Picrotoxinin_Synthesis.png/500px-Picrotoxinin_Synthesis.png)
In 1988, researchers from Tohoku University in Japan completed a total stereoselective synthesis of both (‑)‑picrotoxinin and (-)-picrotin beginning with (+)‑5β‑hydroxycarvone. In this synthesis, eight asymmetric centers were stereoselectively prepared on a cis-fused hydrindane ring system using several different reactions: a Claisen rearrangement to introduce the quaternary center, an organoselenium-mediated reduction of an epoxy ketone, and a stereospecific construction of a glycidic ester.[7]
The June 2020 synthesis instead employed the quick formation of the polycyclic core, followed by the manipulation of oxidation states of key carbon atoms in order to produce the target molecule.[5]
Some research suggests that picritoxin can be made by the cyclofunctionalization of cycloalkenyl systems. Under kinetically controlled conditions, this process generally results in exo cyclization and forms bridged ring systems like those found in picrotoxin.[8]
Several techniques have been developed to isolate picrotoxinin and picrotin individually. Reaction with the nearby cis alcohol is the key obstruction, and can be inhibited by pretreatment (protection) with trifluoroacetic anhydride in pyridine:[9]
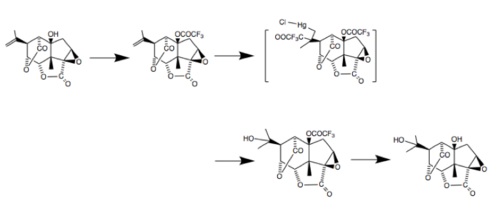
Picrotoxin has also been used as a starting material in several synthetic processes, including the creation of dl-picrotoxadiene, which retains certain features of the picrotoxin skeleton.[10]
Mechanism of action
Some crustacean muscle fibers have excitatory and inhibitory innervation. Picrotoxin blocks inhibition.[11] Two different but related theories have been proposed for the mechanism by which picrotoxin acts on synapses. One theory is that it acts as a non-competitive channel blocker for GABAA receptor chloride channels,[12] specifically the gamma-aminobutyric acid-activated chloride ionophore.[13] A 2006 study found that, while not structurally similar to GABA, picrotoxin prevents ion flow through the chloride channels activated by GABA. It likely acts within the ion channels themselves, rather than at GABA recognition sites. Because it inhibits channels activated by GABA, GABA-enhancing drugs like barbiturates and benzodiazepines can be used as an antidote.[14]
Other research suggests that the toxin acts instead as a non-competitive antagonist, or inhibitor, for GABA receptors. A study by Newland and Cull-Candy found that, in high enough concentrations, picrotoxin reduced the amplitude of GABA currents. Their data indicated that it was unlikely that picrotoxin acted simply as a voltage-gated channel blocker, although it did reduce the frequency of channel openings. Rather, they found that picrotoxin “binds preferentially to an agonist bound form of the receptor.” This means that, even in the presence of low concentrations of picrotoxin, the response of neurons to GABA is reduced.[15]
Toxicity
Picrotoxin acts as a central nervous system and respiratory stimulant. It is extremely toxic to fish and humans, as well as rodents and other mammals. According to the Register of Toxic Effects of Chemical Substances, the LDLo, or lowest reported lethal dose, is 0.357 mg/kg. Symptoms of picrotoxin poisoning include coughing, difficulty breathing, headache, dizziness, confusion, gastro-intestinal distress, nausea or vomiting, and changes in heart rate and blood pressure. Although especially dangerous if swallowed, systemic effects can also result from inhalation or absorption into the blood stream through lesions in the skin.[16] Picrotoxin also acts as a convulsant. In larger doses, it has been found to induce clonic seizures or cardiac dysrhythmias, with especially high doses ultimately proving fatal, typically due to respiratory paralysis.[17]
Clinical applications and other uses
Due to its toxicity, picrotoxin is now most commonly used as a research tool. However, due to its antagonist effect on GABA receptors, it has been used as a central nervous system stimulant. It was also previously used as an antidote for poisoning by CNS depressants, especially barbiturates.[18]
Although not commonly used, picrotoxin is effective as both a pesticide and a pediculicide. In the 19th century, it was used in the preparation of hard multum, which was added to beer to make it more intoxicating. This preparation has since been outlawed.[19][20]
Despite its potential toxicity to mammals in large enough doses, picrotoxin is also sometimes used as a performance enhancer in horses. It is classified as an illegal "Class I substance" by the American Quarter Horse Association. Substances that are classified as “Class I” are likely to affect performance and have no therapeutic use in equine medicine.[21] In 2010, quarter horse trainer Robert Dimitt was suspended after his horse, Stoli Signature, tested positive for the substance. As with humans, it is used to counteract barbiturate poisoning.[22]
See also
References
- ^ Boullay PF (1812). "Analyse chimique de la Coque du Levant, Menispermum cocculus". Bulletin de Pharmacie (in French). 4: 5–34.
Menispermum cocculus" has been renamed "Anamirta cocculus"
- ^ (Boullay, 1812), p. 31.
- ^ Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu Y, et al. "Picrotoxin". DrugBank. Retrieved April 26, 2017.
- ^ Gammill R, Tulinsky J (1994). "The Chemistry and Pharmacology of GABAA and GABAB Ligands". Current Medicinal Chemistry. 1 (3): 242. Retrieved April 26, 2017.
- ^ a b Crossley SW, Tong G, Lambrecht MJ, Burdge HE, Shenvi RA (July 2020). "Synthesis of (-)-Picrotoxinin by Late-Stage Strong Bond Activation". Journal of the American Chemical Society. 142 (26): 11376–11381. doi:10.1021/jacs.0c05042. PMC 8011636. PMID 32573211.
- ^ Trost B, Krische MJ (1996). "Picrotoxinin". Journal of the American Chemical Society. 118: 233. doi:10.1021/ja953060r. Retrieved May 7, 2017.
- ^ Miyashita M, Suzuki T, Yoshikoshi A (May 1989). "Stereoselective total synthesis of (-)-picrotoxinin and (-)-picrotin". Journal of the American Chemical Society. 111 (10): 3728–3734. Bibcode:1989JAChS.111.3728M. doi:10.1021/ja00192a035.
- ^ Trost B, Fleming I (1991). Comprehensive Organic Synthesis (Volume 4 ed.). Oxford, UK: Pergamon Press. p. 373. ISBN 9780080405957. Retrieved May 7, 2017.
- ^ Corey EJ, Pearce HL (1980). "Total Synthesis of Picrotin". Tetrahedron Letters. 21 (19): 1823–1824. doi:10.1016/s0040-4039(00)92789-8.
- ^ Conroy H (June 1952). "Picrotoxin. II. The Skeleton of Picrotoxinin. The Total Synthesis of dl-Picrotoxadiene". Journal of the American Chemical Society. 74 (12): 3046–3051. Bibcode:1952JAChS..74.3046C. doi:10.1021/ja01132a028.
- ^ Van Der Kloot WG, Robbins J, Cooke IM (March 1958). "Blocking by picrotoxin of peripheral inhibition in crayfish". Science. 127 (3297): 521–522. Bibcode:1958Sci...127..521V. doi:10.1126/science.127.3297.521. PMID 13529017.
- ^ Rho JM, Donevan SD, Rogawski MA (December 1996). "Direct activation of GABAA receptors by barbiturates in cultured rat hippocampal neurons". The Journal of Physiology. 497 (2): 509–22. doi:10.1113/jphysiol.1996.sp021784. PMC 1161000. PMID 8961191.
- ^ Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu Y, et al. "Picrotoxin". DrugBank. Retrieved April 26, 2017.
- ^ Olsen RW (April 2006). "Picrotoxin-like channel blockers of GABAA receptors". Proceedings of the National Academy of Sciences of the United States of America. 103 (16): 6081–2. Bibcode:2006PNAS..103.6081O. doi:10.1073/pnas.0601121103. PMC 1458832. PMID 16606858.
- ^ Newland CF, Cull-Candy SG (February 1992). "On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat". The Journal of Physiology. 447: 191–213. doi:10.1113/jphysiol.1992.sp018998. PMC 1176032. PMID 1317428.
- ^ "Picrotoxin" (PDF). Santa Cruz Biotechnology. Retrieved April 26, 2017.
- ^ "Picrotoxin". Toxnet. U.S. National Laboratory of Medicine. Retrieved April 26, 2017.
- ^ Nilsson E, Eyrich B (2009). "On treatment of barbiturate poisoning". Acta Medica Scandinavica. 137 (6): 381–9. doi:10.1111/j.0954-6820.1950.tb12129.x. PMID 15432128.
- ^ Böttger A, Vothknecht U, Bolle C, Wolf A (2018). "Plant-Derived Drugs Affecting Ion Channels". Lessons on Caffeine, Cannabis & Co: Plant-derived Drugs and their Interaction with Human Receptors. Learning Materials in Biosciences. p. 129. doi:10.1007/978-3-319-99546-5_8. ISBN 978-3-319-99545-8.
- ^ Bell J (1869). Report of the Committee on the Relations of Alcohol to Medicine. United States: Collins. p. 32.
- ^ "Uniform Classification Guidelines for Foreign Substances and Recommended Penalties and Model Rule" (PDF). Association of Racing Commissioners International, Inc. Retrieved April 26, 2017.
- ^ Lemoreaux P (September 2, 2017). "Two Quarter Horse trainers suspended for drug violations at Prairie Meadows". Daily Racing Form. Daily Racing Form. Retrieved April 26, 2017.
Further reading
- Ehrenberger K, Benkoe E, Felix D (1982). "Suppressive action of picrotoxin, a GABA antagonist, on labyrinthine spontaneous nystagmus and vertigo in man". Acta Oto-Laryngologica. 93 (1–6): 269–73. doi:10.3109/00016488209130882. PMID 7064710.
- Dupont L, Dideberg O, Lamotte-Brasseur J, Angenot L (1976). "Structure cristalline et moléculaire de la picrotoxine, C15H16O6·C15H18O7". Acta Crystallographica B (in French). 32 (11): 2987–2993. Bibcode:1976AcCrB..32.2987D. doi:10.1107/S0567740876009424. hdl:2268/31560.
- Olsen RW, DeLorey TM (1999). "GABA Receptor Physiology and Pharmacology". In Siegel GJ, Agranoff BW, Albers RW, et al. (eds.). Basic Neurochemistry: Molecular, Cellular and Medical Aspects (6th ed.). Philadelphia, PA, USA: Lippincott-Raven.