Trends in LIMS
Contents
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
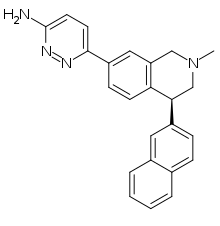
| Formula | C24H22N4 |
| Molar mass | 366.468 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Liafensine (BMS-820836) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) which was under development by Bristol-Myers Squibb for the treatment of major depressive disorder.[1][2] Though it demonstrated comparable effectiveness to escitalopram and duloxetine in phase II clinical trials, development was paused in 2013 because liafensine failed to show superior effectiveness relative to these drugs, a decision that was made likely based on its increased capacity for side effects as well as potential for abuse.[1] Another clinical trial of liafensine began in 2022.[3]
See also
References
- ^ a b "Digest". Progress in Neurology and Psychiatry. 17 (5): 41–43. 2013. doi:10.1002/pnp.305. ISSN 1367-7543. S2CID 222168896.
- ^ Bang-Andersen B, Bøgesø KP, Kehler J, Sánchez C (2017). "New Trends in Antidepressant Drug Research". In Ecker GF, Clausen RP, Sitte HH (eds.). Transporters as drug targets. Methods and Principles in Medicinal Chemistry. Weinheim, Germany: John Wiley & Sons. pp. 21–52 (22). doi:10.1002/9783527679430.ch2. ISBN 978-3-527-33384-4.
- ^ "CTG Labs - NCBI". clinicaltrials.gov. Retrieved 3 December 2023.

















