The US FDA’s proposed rule on laboratory-developed tests: Impacts on clinical laboratory testing
Original file (2,174 × 1,205 pixels, file size: 299 KB, MIME type: image/jpeg)
Summary
| Description |
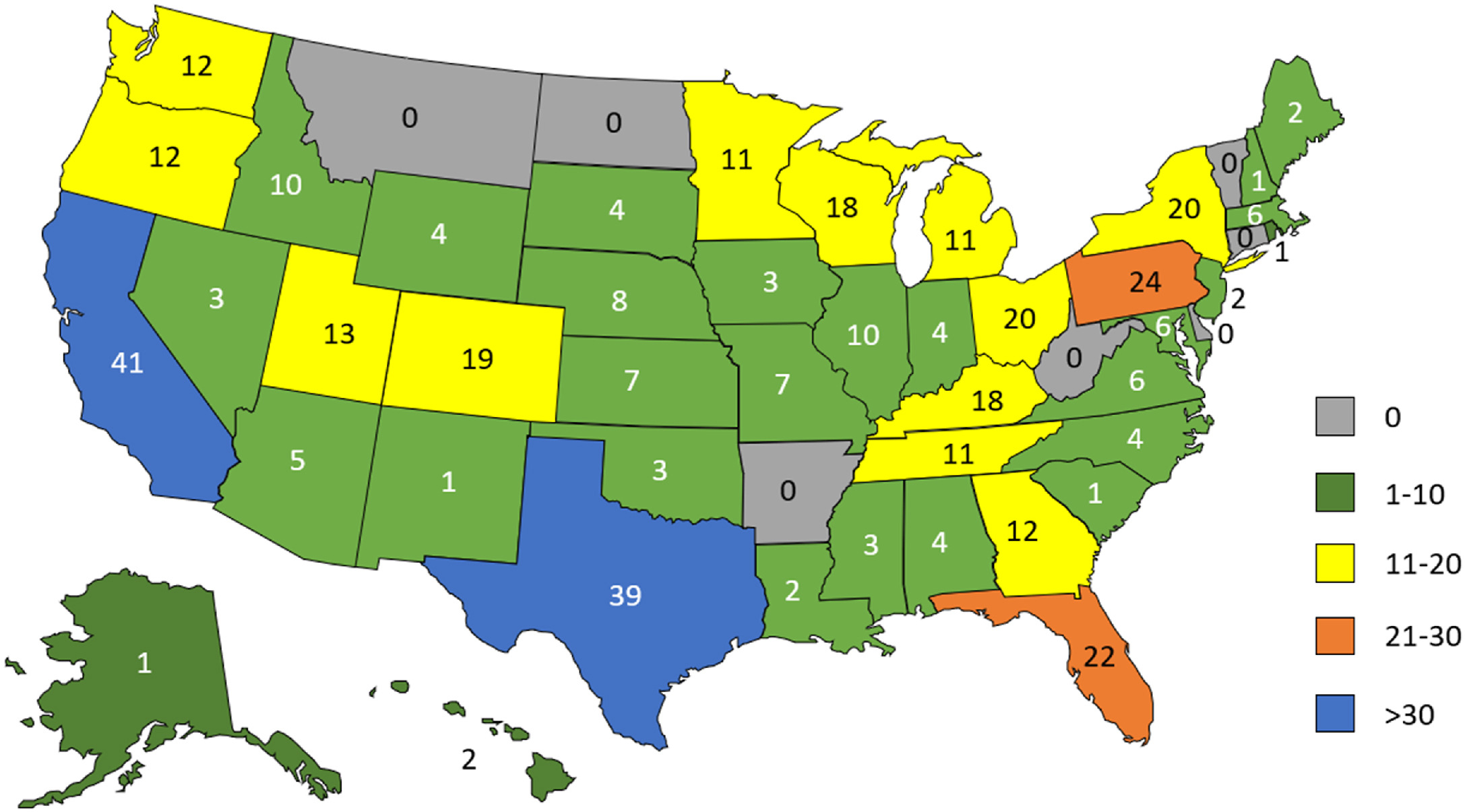
Figure 1. Respondent institution by U.S. state. Map showing respondent institution by state (where available). |
|---|---|
| Source |
Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. (2024). "The US FDA’s proposed rule on laboratory-developed tests: Impacts on clinical laboratory testing". Practical Laboratory Medicine In press: e00407. doi:10.1016/j.plabm.2024.e00407. |
| Date |
2024 |
| Author |
Smith, Leslie; Carricaburu, Lisa A.; Genzen, Jonathan R. |
| Permission (Reusing this file) |
Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International |
| Other versions |
Licensing
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
| current | 19:23, 26 May 2024 |  | 2,174 × 1,205 (299 KB) | Shawndouglas (talk | contribs) |
You cannot overwrite this file.
File usage
The following page uses this file:
















