The US FDA’s proposed rule on laboratory-developed tests: Impacts on clinical laboratory testing
Contents
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌlɪnzəˈɡoʊlɪks/ LINZ-ə-GOH-liks |
| Trade names | Yselty |
| Other names | KLH-2109; OBE-2109 |
| Routes of administration | By mouth[1][2] |
| Drug class | GnRH modulator; GnRH antagonist; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL |
|
| Chemical and physical data | |
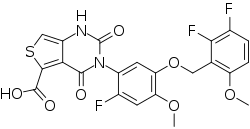
| Formula | C22H15F3N2O7S |
| Molar mass | 508.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Linzagolix, sold under the brand name Yselty, is a medication used in the treatment of uterine fibroids.[1][5] Linzagolix is a small-molecule, non-peptide, orally active gonadotropin-releasing hormone antagonist (GnRH antagonist) developed by Kissei Pharmaceutical and ObsEva.[6][7][2]
In June 2022, it was approved for medical use in the European Union and in the United Kingdom.[1][4][8]
Medical uses
Linzagolix is indicated for treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age.[1]
Available forms
Linzagolix is available as linzagolix choline, the choline salt of linzagolix, in the form of 100 and 200 mg film-coated oral tablets.[5]
Pharmacology
Pharmacodynamics
Linzagolix acts as a selective antagonist of the GnRH receptor, the biological target of GnRH.[5] By blocking this receptor, linzagolix prevents GnRH-mediated secretion of the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and prevents them from signaling the gonads to produce sex hormones including estrogens, progesterone, and androgens.[5][9]
In clinical studies, linzagolix fully suppressed estradiol levels (median <20 pg/mL) in women at a dosage of 200 mg/day, whereas partial suppression of estradiol levels (median 20–60 pg/mL) occurred at a dosage 100 mg/day.[5] Progesterone levels were also variably suppressed with these dosages.[5]
Pharmacokinetics
The elimination half-life of linzagolix with repeated administration is approximately 15 hours.[5]
Society and culture
Legal status
On 16 December 2021, and on 22 April 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Yselty, intended for the treatment of symptoms of uterine fibroids.[10] The applicant for this medicinal product is ObsEva Ireland Ltd.[10] Linzagolix was approved for medical use in the European Union in June 2022.[1][4]
Brand names
Linzagolix is sold under the brand name Yselty.[5]
Availability
Linzagolix is available in the European Union and in the United Kingdom.[5][8]
References
- ^ a b c d e f "Yselty EPAR". European Medicines Agency (EMA). 14 December 2021. Retrieved 19 August 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ a b Ezzati M, Carr BR (January 2015). "Elagolix, a novel, orally bioavailable GnRH antagonist under investigation for the treatment of endometriosis-related pain". Women's Health. 11 (1): 19–28. doi:10.2217/whe.14.68. PMID 25581052.
- ^ "Linzagolix". Specialist Pharmacy Service. 8 August 2022. Retrieved 24 August 2022.
- ^ a b c "Yselty Product information". European Commission. EU/1/21/1606. Retrieved 24 June 2022.
- ^ a b c d e f g h i "Yselty film-coated tablets" (PDF). Theramex Ireland Limited. European Medicines Agency.
- ^ "Linzagolix - Kissei Pharmaceutical/ObsEva". AdisInsight. Springer Nature Switzerland AG.
- ^ Chodankar R, Allison J (2018). "New Horizons in Fibroid Management". Current Obstetrics and Gynecology Reports. 7 (2): 106–115. doi:10.1007/s13669-018-0242-6. ISSN 2161-3303.
- ^ a b "ObsEva Announces UK MHRA Marketing Authorization for Yselty (linzagolix), an Oral GnRH Antagonist, for the Treatment of Uterine Fibroids" (Press release). ObsEva. 28 June 2022. Retrieved 24 August 2022 – via GlobeNewswire.
- ^ Kotlyar AM, Pal L, Taylor HS (December 2021). "Eliminating Hormones With Orally Active Gonadotropin-releasing Hormone Antagonists". Clinical Obstetrics and Gynecology. 64 (4): 837–849. doi:10.1097/GRF.0000000000000664. PMID 34668887. S2CID 239034628.
- ^ a b "Yselty: Pending EC decision". European Medicines Agency. 16 December 2021. Retrieved 18 December 2021. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
Further reading
- Dababou S, Garzon S, Laganà AS, Ferrero S, Evangelisti G, Noventa M, et al. (September 2021). "Linzagolix: a new GnRH-antagonist under investigation for the treatment of endometriosis and uterine myomas". Expert Opinion on Investigational Drugs. 30 (9): 903–911. doi:10.1080/13543784.2021.1957830. PMID 34278887. S2CID 236091261.
External links
- "Linzagolix". Drug Information Portal. U.S. National Library of Medicine.
- "Linzagolix choline". Drug Information Portal. U.S. National Library of Medicine.

















