The US FDA’s proposed rule on laboratory-developed tests: Impacts on clinical laboratory testing
Contents
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3,5-Triazinane-2,4,6-trione[1] | |||
| Other names
1,3,5-Triazine-2,4,6(1H,3H,5H)-trione[1]
1,3,5-Triazinetriol s-Triazinetriol s-Triazinetrione Tricarbimide Isocyanuric acid Pseudocyanuric acid | |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.290 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C3H3N3O3 | |||
| Molar mass | 129.07 g/mol | ||
| Appearance | white crystalline powder | ||
| Density | 1.75 g/cm3 | ||
| Melting point | 320–360 °C (608–680 °F; 593–633 K) decomposes | ||
| 2700 mg/L (25 °C) | |||
| -61.5·10−6 cm3/mol | |||
| Hazards | |||
| Safety data sheet (SDS) | ICSC 1313 | ||
| Related compounds | |||
Related triazines
|
Cyanuric fluoride Cyanuric chloride Cyanuric bromide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Cyanuric acid or 1,3,5-triazine-2,4,6-triol is a chemical compound with the formula (CNOH)3. Like many industrially useful chemicals, this triazine has many synonyms. This white, odorless solid finds use as a precursor or a component of bleaches, disinfectants, and herbicides. In 1997, worldwide production was 160 000 tonnes.[2]
Properties and synthesis
Properties
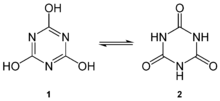
Cyanuric acid can be viewed as the cyclic trimer of the elusive chemical species cyanic acid, HOCN. The ring can readily interconvert between several structures via lactam–lactim tautomerism. Although the triol tautomer may have aromatic character, the keto form predominates in solution.[3] The hydroxyl (-OH) groups assume phenolic character. Deprotonation with base affords a series of cyanurate salts:
- [C(O)NH]3 ⇌ [C(O)NH]2[C(O)N]− + H+ (pKa = 6.88)[4]
- [C(O)NH]2[C(O)N]− ⇌ [C(O)NH][C(O)N]22− + H+ (pKa = 11.40)
- [C(O)NH][C(O)N]22− ⇌ [C(O)N]33− + H+ (pKa = 13.5)
Cyanuric acid is noted for its strong interaction with melamine, forming insoluble melamine cyanurate. This interaction locks the cyanuric acid into the tri-keto tautomer. Melamine cyanurate is cited as an example of supramolecular chemistry.[5]
Synthesis
Cyanuric acid (CYA) was first synthesized by Friedrich Wöhler in 1829 by the thermal decomposition of urea and uric acid.[6] The current industrial route to CYA entails the thermal decomposition of urea, with release of ammonia. The conversion commences at approximately 175 °C:[2]
- 3 H2N-CO-NH2 → [C(O)NH]3 + 3 NH3
CYA crystallizes from water as the dihydrate.
Cyanuric acid can be produced by hydrolysis of crude or waste melamine followed by crystallization. Acid waste streams from plants producing these materials contain cyanuric acid and on occasion, dissolved amino-substituted triazines, namely, ammeline, ammelide, and melamine. In one method, an ammonium sulfate solution is heated to the "boil" and treated with a stoichiometric amount of melamine, by which means the cyanuric acid present precipitates as melamine-cyanuric acid complex. The various waste streams containing cyanuric acid and amino-substituted triazines may be combined for disposal, and during upset conditions undissolved cyanuric acid may be present in the waste streams. [7][8]
Intermediates and impurities
Intermediates in the dehydration include both isocyanic acid, biuret, and triuret:
- H2N-CO-NH2 → HNCO + NH3
- H2N-CO-NH2 + HNCO → H2N-CO-NH-CO-NH2
- H2N-CO-NH-CO-NH2 + HNCO → H2N-CO-NH-CO-NH-CO-NH2
As temperature exceeds 190 °C, other reactions begin to dominate the process.
The first appearance of ammeline occurs prior to 225 °C and is suspected also to occur from decomposition of biuret but is produced at a lower rate than that of CYA or ammelide.
- 3 H2N-CO-NH-CO-NH2 → [C(O)]2(CNH2)(NH)2N + 2 NH3 + H2O
Melamine, [C(NH2)N]3, formation occurs between 325–350 °C and only in very small quantities.[9]
N-substituted isocyanurates from isocyanates
N-substituted isocyanurates can be synthesised by the trimerisation of isocyanates. This is utilised industrially in the formation of polyisocyanurates.
Applications
Cyanuric acid is used as a chlorine stabilizer / buffer in swimming pools. It binds to free chlorine and releases it slowly, extending the time needed to deplete each dose of sanitizer. A chemical equilibrium exists between the acid with free chlorine and its chlorinated form.[10]
Precursors to chlorinated cyanurates
Cyanuric acid is mainly used as a precursor to N-chlorinated cyanurates, which are used to disinfect water. The dichloro derivative is prepared by direct chlorination:
- [C(O)NH]3 + 2 Cl2 + 2 NaOH → [C(O)NCl]2[C(O)NH] + 2 NaCl + 2 H2O
This species is typically converted to its sodium salt, sodium dichloro-s-triazinetrione. Further chlorination gives trichloroisocyanuric acid, [C(O)NCl]3.[2]
These N-chloro compounds serve as disinfectants and algicides for swimming pool water.[2] The aforementioned equilibrium stabilizes the chlorine in the pool and prevents the chlorine from being quickly consumed by sunlight.[10]
Precursors to crosslinking agents
Because of its trifunctionality, CYA is a precursor to crosslinking agents, especially for polyurethane resins and polyisocyanurate thermoset plastics.
The experimental antineoplastic drug teroxirone (triglycidyl isocyanurate) is formed by reacting cyanuric acid with 3 equivalents of epichlorohydrin. It works by cross-linking DNA.[11]
Analysis
Testing for cyanuric acid concentration is commonly done with a turbidometric test, which uses a reagent, melamine, to precipitate the cyanuric acid. The relative turbidity of the reacted sample quantifies the CYA concentration. Referenced in 1957, this test[12] works because melamine combines with the cyanuric acid in the water to form a fine, white precipitate of the insoluble complex melamine cyanurate that causes the water to cloud in proportion to the amount of cyanuric acid in it. More recently, a sensitive method has been developed for analysis of cyanuric acid in urine.[13]
Animal feed
FDA permits a certain amount of cyanuric acid to be present in some non-protein nitrogen (NPN) additives used in animal feed and drinking water.[14] Cyanuric acid has been used as NPN. For example, Archer Daniels Midland manufactures an NPN supplement for cattle, which contains biuret, triuret, cyanuric acid and urea.[15]
2007 pet food recalls
Cyanuric acid is implicated in connection to the 2007 pet food recalls, the contamination and wide recall of many brands of cat and dog foods beginning in March 2007. Research has found evidence that cyanuric acid, a constituent of urine, together with melamine forms poorly soluble crystals which can cause kidney failure (see Analysis section above).
Safety
Cyanuric acid is classified as "essentially nontoxic".[2] The 50% oral median lethal dose (LD50) is 7700 mg/kg in rats.[16]
However, when cyanuric acid is present together with melamine (which by itself is another low-toxicity substance), it will form an insoluble and rather nephrotoxic complex,[17] as evidenced in dogs and cats during the 2007 pet food contamination and in children during the 2008 Chinese milk scandal cases.
Natural occurrence
An impure copper salt of the acid, with the formula Cu(C3N3O3H2)2(NH3)2, is currently the only known isocyanurate mineral, called joanneumite. It was found in a guano deposit in Chile. It is very rare.[18]
References
- ^ a b Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 733. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b c d e Klaus Huthmacher, Dieter Most "Cyanuric Acid and Cyanuric Chloride" Ullmann's Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. doi 10.1002/14356007.a08 191
- ^ Pérez-Manríquez, Liliana; Cabrera, Armando; Sansores, Luis Enrique; Salcedo, Roberto (7 September 2010). "Aromaticity in cyanuric acid". Journal of Molecular Modeling. 17 (6): 1311–1315. doi:10.1007/s00894-010-0825-2. PMC 3102184. PMID 20820829.

- ^ "Dissociation constants of organic acids and bases" CRC Handbook of Chemistry and physics, Internet Version 2005 (85th ed.)
- ^ Lehn, J. M. (1995). Supramolecular Chemistry: Concepts and Perspectives. Weinheim: VCH.
- ^ Wöhler, F. (1829) "Ueber die Zersetzung des Harnstoffs und der Harnsäure durch höhere Temperatur," (On the decomposition of urea and uric acid at higher temperature), Annalen der Physik und Chemie, 2nd series, 15 : 619-630.
- ^ "Process for preparing pure cyanuric acid". July 14, 1981. Retrieved 2007-12-10.
- ^ "High pressure thermal hydrolysis process to decompose triazines in acid waste streams". March 22, 1977. Retrieved 2007-12-10.
- ^ Shaber, Peter M.; et al. (August 1999). "Study of the thermal decomposition of urea (pyrolysis) reaction and importance to cyanuric acid production" (PDF). American Laboratory: 13–21. Archived from the original (PDF) on 2007-09-28. Retrieved 2007-05-08.
- ^ a b "What Is Pool Stabilizer". 2021-05-18. Retrieved 2022-07-27.
- ^ M. Budnowski, Angew. Chem., 7, 827 (1968).
- ^ "Merck Turbidity Test". Merck. June 6, 2003. Archived from the original on July 1, 2007. Retrieved 2007-05-06. (dead link 8 April 2018)
- ^ Panuwet P, Wade EL, Nguyen JV, Montesano MA, Needham LL, Barr DB. Quantification of cyanuric acid residue in human urine using high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2010 878(28):2916-2922.
- ^ "21CFR573.220 Feed-grade biuret". U.S. Food and Drug Administration. April 1, 2006. Retrieved 2007-05-06.
- ^ "Roughage Buster Plus: ingredients". Archer Daniels Midland. Archived from the original on 2007-02-12. Retrieved 2007-05-06.
- ^ U.S. Food and Drug Administration, "Interim Melamine and Analogues Safety/Risk Assessment; Availability", Archived December 16, 2007, at the Wayback Machine Federal Register: May 30, 2007 (Volume 72, Number 103). Accessed 2008-09-27.
- ^ "Melamine and Cyanuric Acid Interaction May Play Part in Illness and Death from Recalled Pet Food" Archived May 18, 2007, at the Wayback Machine, American Veterinary Medical Association (AVMA), Press Release, May 1, 2007. Accessed 2008-09-27.
- ^ Mindat, http://www.mindat.org/min-42755.html
External links
- International Chemical Safety Card 1313
- Oregon Veterinary Medical Association (OVMA) Pet Food Contamination Page Archived 2008-10-20 at the Wayback Machine – News and developments updated regularly