The cannabis terpenes
| Full article title | The cannabis terpenes |
|---|---|
| Journal | Molecules |
| Author(s) | Sommano, Sarana R.; Chittasupho, Chuda; Ruksiriwanich, Warintorn; Jantrawut, Pensak |
| Author affiliation(s) | Chiang Mai University |
| Primary contact | Email: sarana dot s at cmu dot ac dot th |
| Editors | Valgimigli, Luca |
| Year published | 2020 |
| Volume and issue | 25(24) |
| Article # | 5792 |
| DOI | 10.3390/molecules25245792 |
| ISSN | 1420-3049 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.mdpi.com/1420-3049/25/24/5792/htm |
| Download | https://www.mdpi.com/1420-3049/25/24/5792/pdf (PDF) |
Abstract
Terpenes are the primary constituents of essential oils and are responsible for the aroma characteristics of the Cannabis plant. Together with cannabinoids, terpenes illustrate a potential synergic and/or entourage effect, with their interactions having only been speculated on for the last few decades. Hundreds of terpenes have been identified that additionally add to the overall cannabis sensory experience, contributing largely to the consumer’s experiences, as well as the market price. These terpenes also enhance many therapeutic efforts, especially as aromatherapy. To shed light on the importance of terpenes in the cannabis industry, the purpose of this review is to morphologically describe sources of cannabis terpenes and to explain the biosynthesis and diversity of terpene profiles in different cannabis chemotypes.
Introduction
Cannabis sativa L. or Cannabis is a herbaceous annual that has a long history of use around the world as fiber, food, oil, and medicine. Depending on the purpose of utilization, cannabis can be called by different names, for example, “hemp” as a fiber and textile crop, and “recreational cannabis,” also known in the United States as "marijuana." Aside from its quality as an industrial textile, the psychoactive properties have placed a stigma on Cannabis plants as an illicit drug, with many informal names including pot, dope, grass, weed, Mary Jane, bud, hash, bhang, kef, ganja, locoweed, reefer, doob, spliff, toke, and roach. It has been forbidden to grow in many countries due to its psychoactive constituents.[1] From a medical perspective, many recent studies have advised that an increase in cannabis use is associated with psychiatric symptoms, including depression and anxiety.[2][3] However, many users still exclusively endorse its recreational purpose.[4][5] As a result, there has been a strong movement toward correcting negative attitudes about Cannabis, and attempts have been made in trying to remove this plant from narcotic lists. In Thailand, for instance, as of 2020, cannabis strains such as กัญชง (hemp) and กัญชา (marijuana) are legally grown for industrial fiber or medicinal purposes based on the controlled level of active metabolites, including cannabinoids such as Δ9–tetrahydrocannabinol (THC) and cannabidiol (CBD).[6] While primary focus has been paid to the bioactive functions of the cannabinoids, the hydrocarbon terpenes could also potentially offer interesting entourage effects that could ideally synergize or downstream their effects.[7] Eminently, with the rise in the legal cannabis industry, interest has grown around cannabis terpenes as they contribute many of the different aromatic characteristics that influence the diverse varieties of cannabis strains.[8]
Within the scope of this review, we provide the general background history of cannabis discovery and the importance of terpenes. The taxonomy and morphology of cannabis, particularly in regards to the localization of its terpenes, are discussed. More importantly, the chemistry, biosynthesis, and diversity of terpenes in different cannabis genotypes are of major interest in this review.
The cannabis discovery and its importance as a source of terpene
Cannabis has a long history dating back approximately to just after the Ice Age, as cord and textile scraps made of cannabis fiber have been found in historic caves in the Czech Republic.[9][10] In the nineteenth century, the plant was recorded as originating near the southern area of the Caspian Sea near Iran (Figure 1b).[11][12] It was later confirmed by the chemotaxonomy of the essential oil from cannabis of diverse origins, and most of the cannabis phenotypes collected around the globe had chemical ingredients similar to those of Central Asian origin.[13] In previous days, it was known as the original fiber plant in Asian culture. Seed and seed oil extracts were also used as food.[11][14] The first record of cannabis in the literature in China can be dated to approximately 5,000 years ago, as written by emperor Chen Nong, who was then known as the father of Chinese agriculture. The Chinese alphabet's “Ma” was created using the mimic of the cannabis drying process (Figure 1a). The letter was adjusted to describe the male plant, “his” separated from the female plant, “chu” for the quality of the fiber.[15][16] In 500 BC, the use of cannabis spread worldwide from Asia to Europe and to Africa through the silk road. In the nineteenth century, hemp was popular in the western world as a fiber crop that had superior qualities.[17][18] It is not too surprising therefore that cannabis is today known as an ideal plant in terms of sustainability. In a period of rapid industrial growth, hemp became the industrial crop as countries raced against one another toward modernity. In addition to the world textile industry, the plant began to be known for its medical uses. It was believed that during the nineteenth century, there were thousands of cannabis medicines available, produced by more than 280 manufacturers.[19] However, the growth and interest in this fiber crop crashed after the attempt to add it to the narcotic list with opium during the 1925 International Opium Convention in Geneva.[19]
|
The first cannabinoid isolates used for medicinal purposes were produced in Czechoslovakia, and CBD was fully characterized for the first time in 1963, followed by the psychoactive THC in the following year.[20][21][22] The discovery of cannabinoid receptors, CB1 and CB2, together with the full comprehension of the endocannabinoid system, helped us recognize the medicinal benefits of this plant.[23][24] In 1942, Simonsen and Todd[25] were the first researchers to separate terpene fractions from cannabinoids in the literature, and p-Cymene was reported as a main constituent from Egyptian hashish. Only in the past years have the terms "synergic" and "entourage effect" appeared in speculations by chemists all over the world in relation to cannabis compounds, included the terpenes.[26] The proposed synergies were first described as routes for the molecular regulation of endogenous cannabinoid activity.[27] Russo[28] later proposed the unique therapeutic effect of cannabis terpenes that possibly play a role on the entourage effects of the medicinal properties of the cannabinoids. This phytocannabinoid-terpenoid synergy could potentially enhance the treatments of pain, inflammation, depression, anxiety, addiction, epilepsy, cancer, fungal, and bacterial infections.[7][28][29][30][31]
Taxonomy and localization of cannabis terpenes
Cannabis belongs to the small family of flowering plants known as Cannabaceae, which includes hop and Celtis berry. It is a dicotylate angiosperm that gives incomplete (lacking of petals) and also imperfect flower types with the separated sexual organs. The flower bears only pistils, known as pistillate flowers (or female flowers), and those with only the stamens are called staminate (or male flowers). In nature, Cannabis produces either male or female flowers (dioecious); however, under short-day conditions, it may give both male and female flowers or monoecious. Plants are able to grow as high as three meters, or smaller depending on the varieties and the conditions of growth. The flowers often initiate as groups of flowers or as an axillary bud (Figure 2a and 2b). The stem consists of the outermost layer of the epidermis, which is thin and coarse, and the primary and secondary layers that provide the better fiber quality. The innermost, which is the lignified secondary blast fiber, give the best fiber quality.[32][33]
|
Leaves are palmate with five to seven leaflets. The male flower (Figure 3a) has no petals, usually with five yellowish tepals, and five anthers yielding pollen. The female flower (Figure 3b) had a single-ovulate and is enriched with trichome structures, which are the localization points of cannabinoids and terpenes, as shown in Figure 3c. These terpenes are responsible for the defense and interaction with herbivores and pests.
|
Taxonomists classified Cannabis plants into three species in the early days: (i) C. sativa Linnaeus, (ii) C. indica Lamarck, and (iii) C. ruderalis.[34][35] Today, many researchers are convinced that Cannabis that grows commercially is C. sativa L., but the subvariety “sativa” should be known as hemp and the subvariety “indica” should be called recreational cannabis or marijuana. The differences in these sub-varieties are shown in Table 1.
| |||||||||||||||||||||||||||
The most usable part for hemp is the stem in particular, while parts usually with trichomes are the most usable for cannabis. The level of THC is graded as >2% of dry weight, and flowers give a much higher terpene content, which becomes sticky to the touch.[36] Some researchers do not agree with the separation of the two by the chemical compositions. Morphologically, the leaf of cannabis is broader and the color is darker compared to that of hemp. Many recent studies have attempted to separate the combinations of terpene composition in several species of Cannabaceae where it is apparent that hemp and the Indica cannabis are closely related. For example, hemp can also yield terpene profiles similar to those of marijuana.[37]
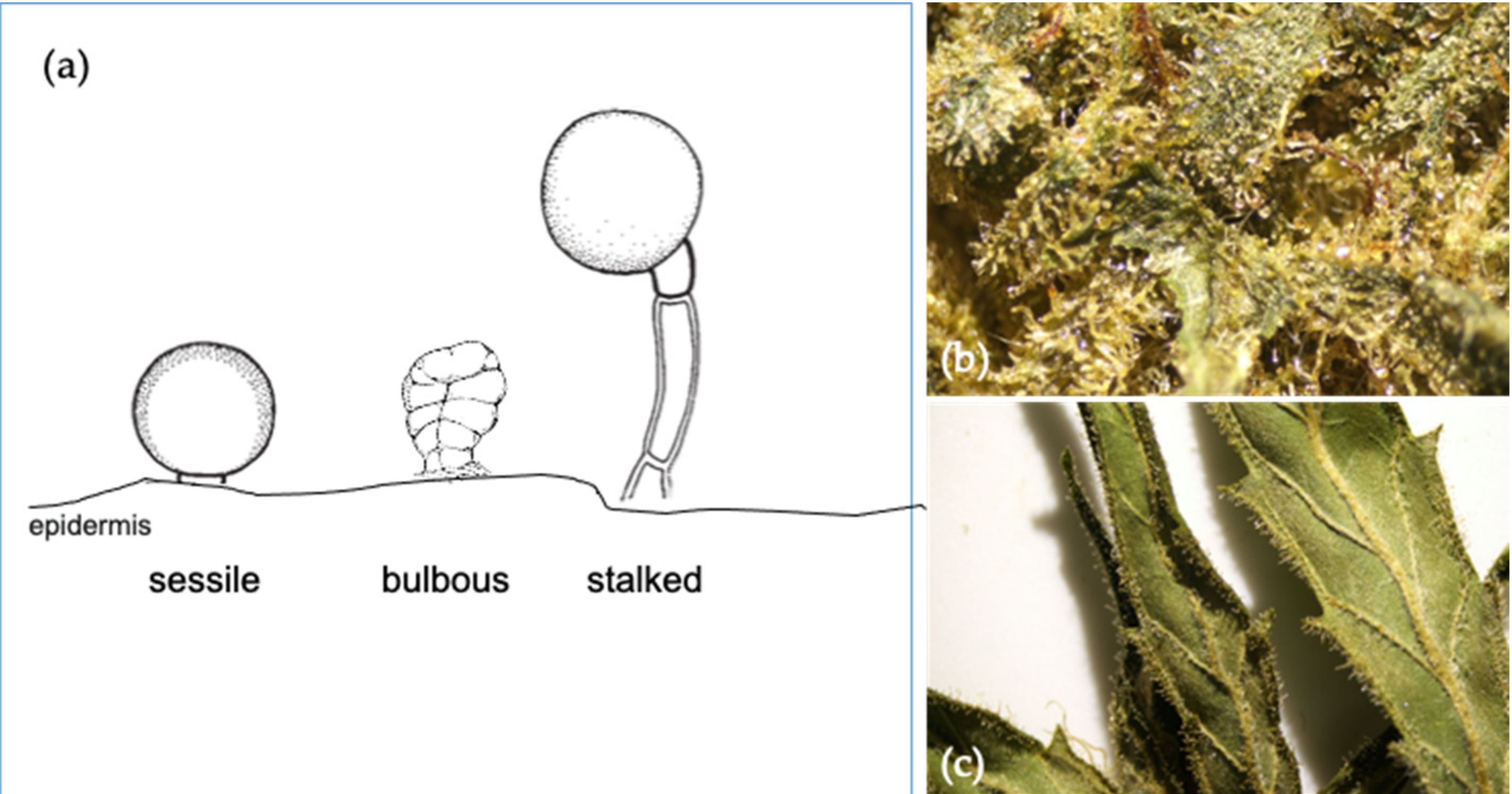
Three types of glandular trichomes are characterized based upon their surface morphology, namely bulbous, sessile, and stalked (Figure 4a).[38] Bulbous trichomes are the smallest, while sessile trichomes appear on the epidermis with a short stalk and a globose head comprised of a multicellular disc of secretory cells and a subcuticular metabolite storage cavity. Similarly, the stalked trichomes are slightly larger with a globose head elevated above the epidermal surface by a multicellular stalk.[39] In cannabis, the sessile and stalked trichomes differ not only in morphology, but they also have distinct fluorescent properties, number of cells in their secretory disc, and terpene profiles.[40] The stalked glandular trichomes of mature flowers have a globular head consisting of an enlarged disc greater than eight secretory cells known to be rich in cannabinoids and monoterpenes (Figure 4b). The sessile trichomes are mainly found on sugar leaves (Figure 4c). They have eight secretory cells that produce fewer cannabinoids and higher proportions of sesquiterpenes.
|
To separate the trichomes, the flowers are usually pre-frozen or freeze-dried and then are gently rubbed on sieve mesh. The trichomes separated in this process are known as kief, which can be pressed to make hash. In Nepal, hash is hand-shaped into balls, also known as wax or “Charas.”[41] Hash oil, on the other hand, is the concentrated hash that has been dissolved in organic solvents such as alcohol, propane, or butane.[42][43] The extraction allows pigments such as chlorophylls and other contaminants to be extracted along with the terpenes, resulting in a dark green color extract. After extraction, the solvent is then removed by evaporation either by direct heat or under a vacuum, resulting in the oil product with high viscosity.
Terpene biosynthesis in cannabis
The energy required for plant growth and development derives from photosynthesis, respiration, and transpiration with O2, CO2, nutrients, and water. The energy is restored in the form of primary chemical ingredients that plants later exploit. These primary metabolites include carbohydrates, lipids, proteins, and nucleic acids. However, during cycles of growth and reproduction, plants might be challenged by stresses including hard environmental conditions, pests, and herbivores. Plants then produce different groups of compounds called secondary metabolites that are used as defenses to those challenges. For example, it can produce compounds that draw in pollinators, including birds to help them in the fertilization process or seed dispersion.[44] These compounds are produced in different forms and are exploited for their biological functionalities[45]; for example, alkaloids such as morphine and codeine in opium give psychoactive and pain relief activity to mammals. Phenolics and flavonoids found in the skins of fruits and berries possess antioxidant activity.[46] Sulfur containing compounds such as allicin in garlic can be used to reduce lipoglycerides in the blood and also have the ability to stimulate appetite.[47] Saponin glycoside in soap nuts can be used as a surfactant[48], and finally, the terpenoids, which are main ingredients found in plants containing essential oils[49], are used as food additives, and some depict psychoactive ability and aroma characteristics such as those found in cannabis.
Terpenes are hydrocarbons with small isoprene units linked to one another to form chains, while terpenoids are oxygen-containing terpenes. Four types of terpenes/terpenoids are usually found in the Cannabis plant[26]:
- monoterpenes (10C) of two isoprene units
- sesquiterpenes (15C) of three isoprene units
- diterpenes (20C) of four isoprene units
- triterpenes (30C) of six isoprene units
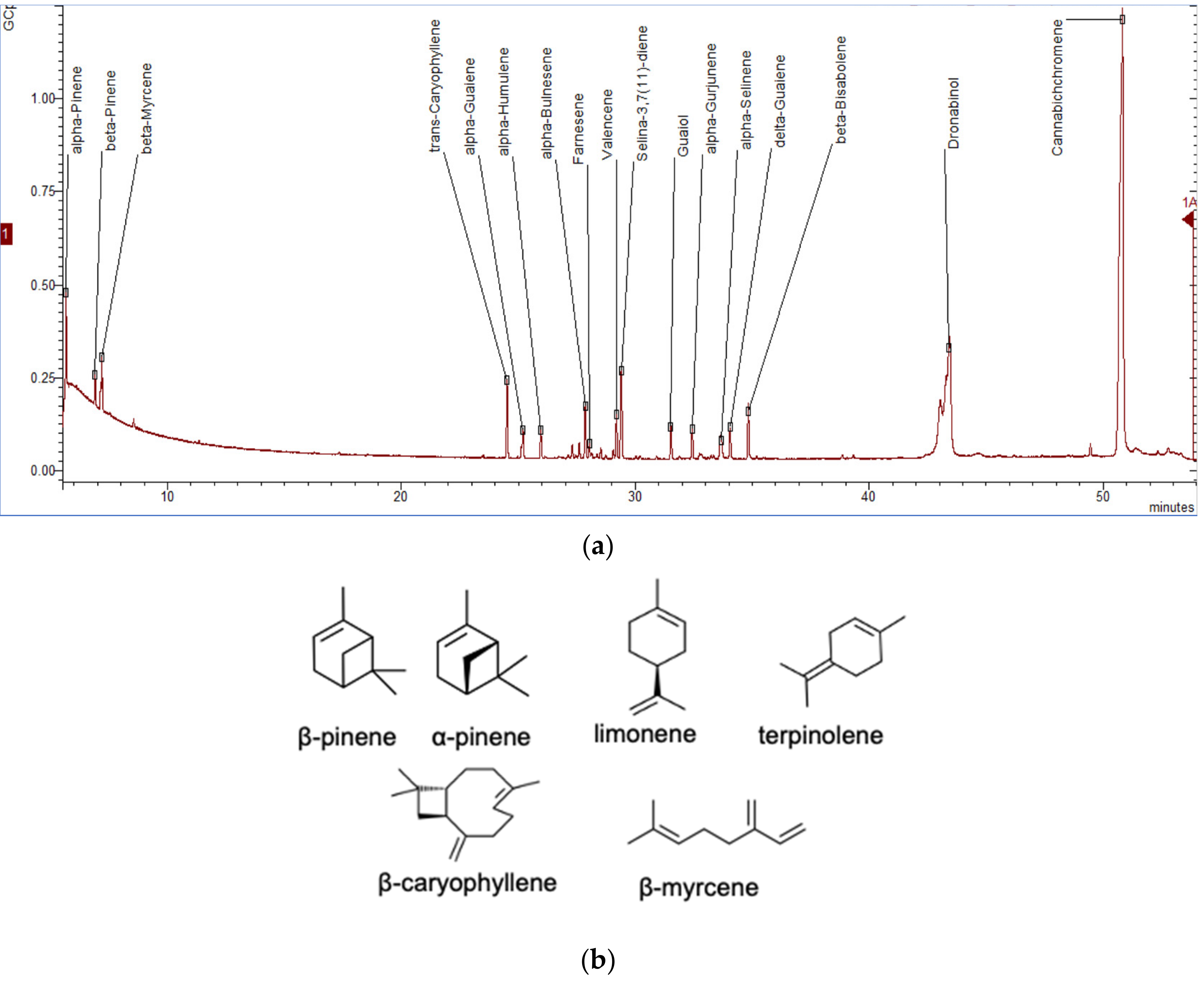
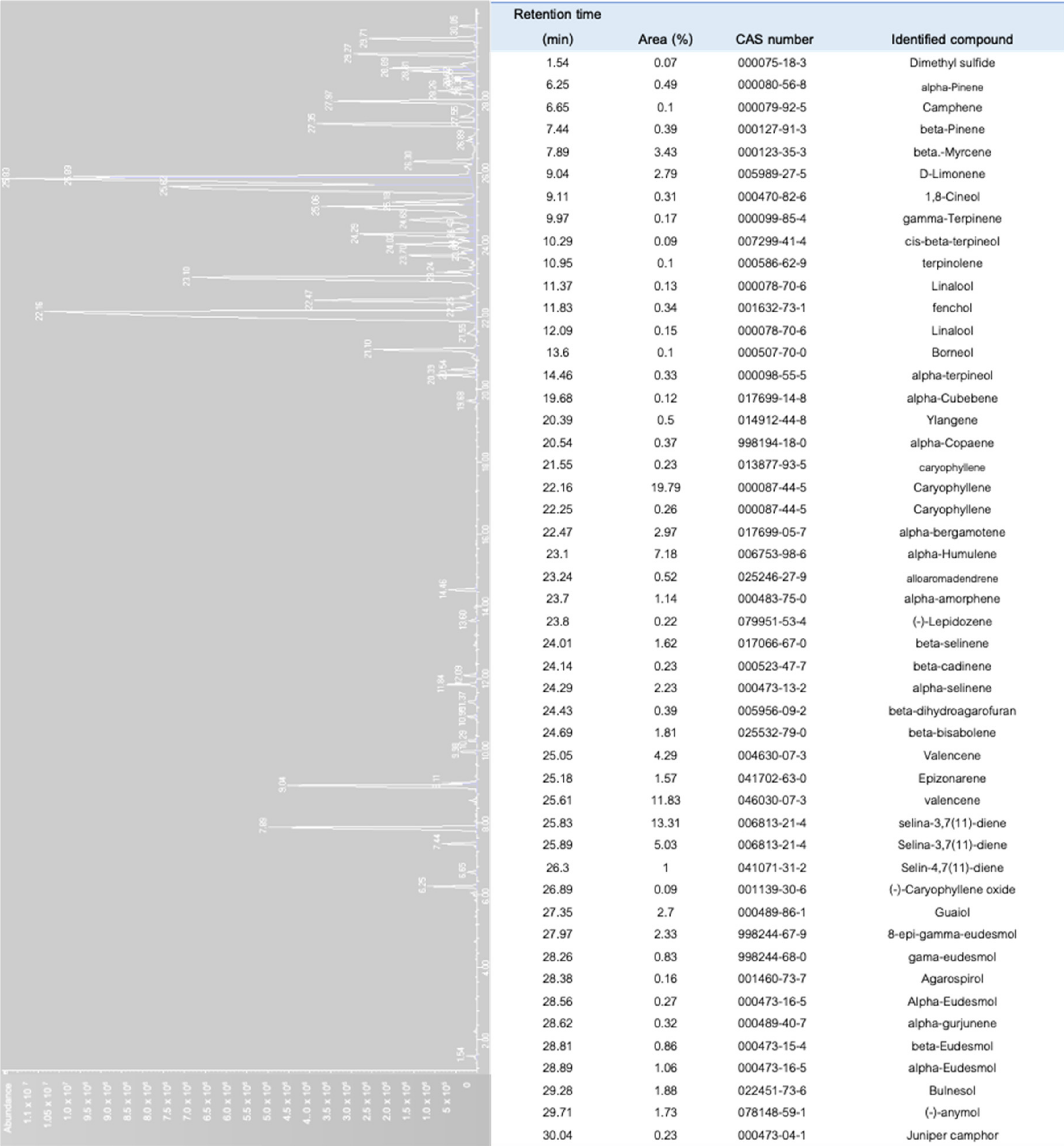
To date, more than 200 volatiles have been reported from the different cannabis genotypes, of which 58 monoterpenes and 38 sesquiterpenes have been characterized.[50][51][52][53] Figure 5a illustrates a chromatogram of the terpene extract from the floral tissue of cannabis. Among others, the major monoterpene components are limonene, β-myrcene, α-pinene, and linalool, with traces of α-terpinolene and tran-ocimene[54][55] (Figure 5b), while predominate sesquiterpenes are E-caryophyllene, caryophyllene oxide, E-β-farnesene, and β-caryophyllene.[56] The cannabinoids are biologically synthesized from diterpene structures to form phenol terpenoids, which account for almost a quarter of all metabolites.[26] Thus, the combination of the terpenes provides the unique aromas to different strains.
|
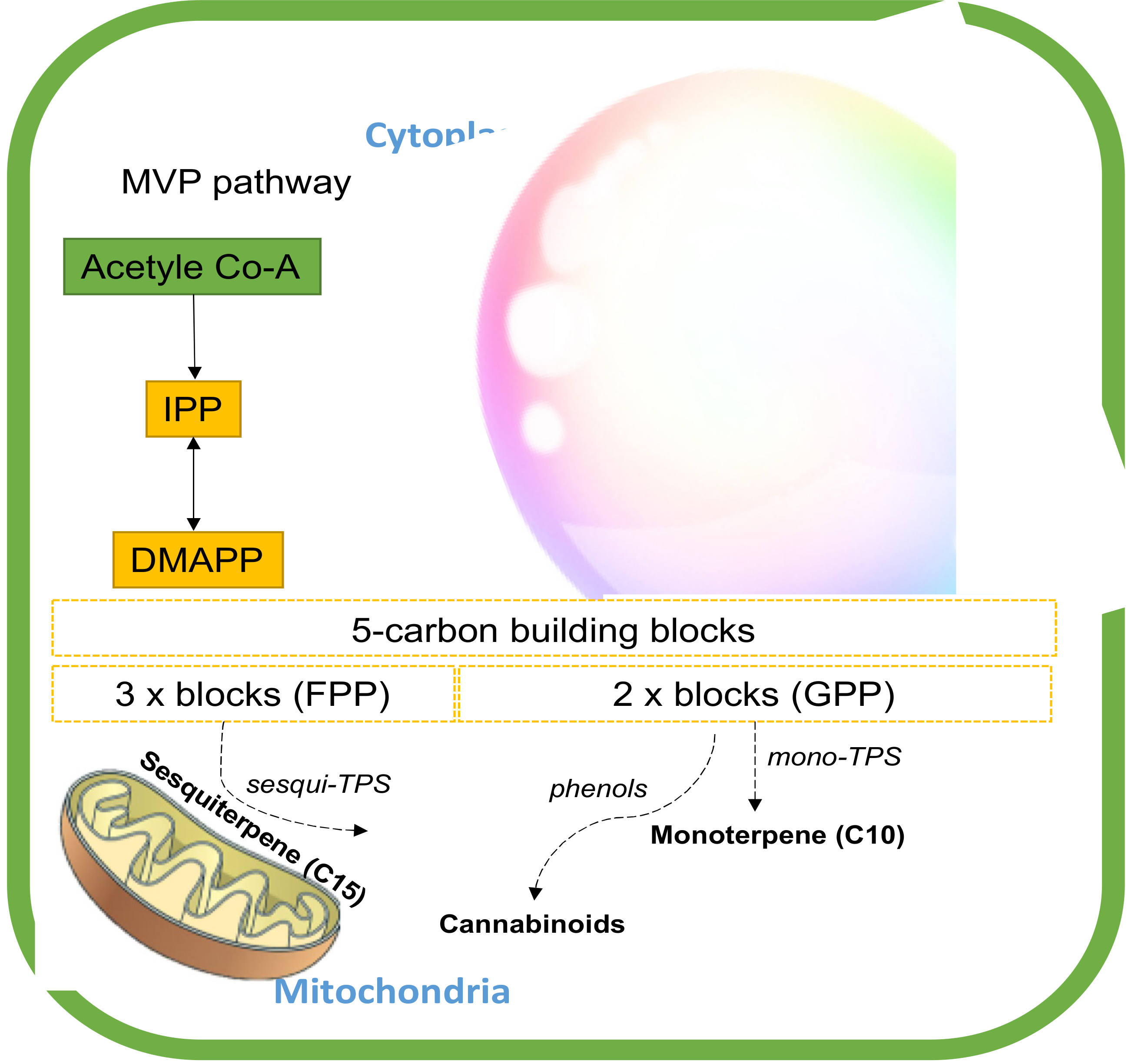
The biosynthesis of these secondary-metabolite terpenes starts with the common isoprenoid diphosphate precursors (5C) through two paths, the plastidial methylerythritol phosphate (MEP) pathway and the cytosolic mevalonate (MEV) pathway.[8][58] These pathways regulate the different substrates available for terpene synthesis (TPS). The MEP converts pyruvate and glyceraldehyde-3-phosphate (G3P) into five-carbon building blocks, isopentenyl diphosphate (IPP), and dimethylallyl diphosphate (DMAPP) in the plastids.[59] The MEV pathway, on the other hand, alters three units of acetyl-CoA to IPP, which is then isomerized to DMAPP by IPP isomerase in cytosol. IPP and DMAPP are condensed into longer-chain isoprenoid diphosphates that include geranyl diphosphate (GPP) and farnesyl diphosphate (FPP).[58] These linear isoprenoid diphosphates are substrates for monoterpene synthases (mono-TPS) and sesquiterpene synthases (sesqui-TPS), respectively, which diversify these precursors through enzymatic modifications such as hydroxylation, dehydrogenation, acylation, and glycosylation into the diverse ranges of mono- and sesquiterpenes.[60][61] GPP is also a building block of cannabinoid biosynthesis (Figure 6).[61]
|
The biosynthetic pathway of cannabinoids involves the chemical joining process of the phenol with the terpenes to form the non-activate acidic forms (that largely determine their potency and pharmaceutical properties), including cannabichromene (CBC), cannabidiolic acid (CBDA), cannabigerol (CBG), cannabinol (CBN), cannabidivarin (CBDV), cannabidivarinic acid (CBDVA), cannabigerolic acid (CBGA), cannabicyclol (CBL), Δ8–tetrahydrocannabinol, tetrahydrocannabinolic acid (THCA), and tetrahydrocannabivarin (THCV).[62] These compounds, along with terpenes, are produced in the trichome structures available on the female cannabis flower.[40] The highest concentration of the natural cannabinoids in cannabis are cannabidiolic acid (CBDA) and Δ9-tetrahydrocannabinoic acid (Δ9-THCA). The psychoactive metabolites such as Δ9–tetrahydrocannabinol and the non-psychoactive CBD are then activated through decarboxylation by heat treatments. It is also favored by several factors such as storage time and the use of alkaline conditions.[63][64]
The following subsections discuss the important cannabis terpene groups and their synergistic and functional properties.
Cannabis monoterpenes
The monoterpenes α-pinene and β-pinene inhibit the activity of acetylcholinesterase in the brain. Therefore, it is claimed to aid memory and minimize cognitive dysfunction induced by THC intoxication.[65] The characteristic of pine scent possesses antiseptic activity.[49][66][67] β-myrcene is known to have the analgesic effect of THC and CBD by stimulating the release of endogenous opioids through the α2-adrenergic receptor dependent mechanism.[68][69] Thus, if the level of myrcene is >0.5%, it may result in a “couch lock” effect, while low levels of myrcene (>0.5% myrcene) can produce a higher level of energy.[26] This compound offers a musky or hop-like fragrance with the functions of antioxidant and anticarcinogens.[28][66] Even though it has been postulated that limonene, with its citrus aroma, has a low affinity for cannabinoid receptors, this monoterpene boosts up the level of serotonin and dopamine, thereby inducing the anxiolytic, anti-stress, and sedative effects of CBD.[68][70] The floral fragrance of linalool could assist with anxiety through aromatherapy.[66]
Cannabis sesquiterpenes
β-Caryophyllene, with a spicy (pepper) aroma, is the most available sesquiterpenoid in Cannabis plants and extracts, especially after decarboxylation. It is an agonist with the CB2 receptor, without psychoactivity.[52] It is also responsible for the anti-inflammatory effects of cannabis.[66] This sesquiterpene is also proven to give gastroprotective, analgesic, anticancerogenic, antifungal, antibacterial, antidepressant, anti-inflammatory, antiproliferative, antioxidant, anxiolytic, analgesic, and neuroprotective effects.[26] Caryophyllene oxide, which gives off a lemon balm-like scent, is proven to have anti-fungal and insecticidal properties.[28]
The cannabis chemovars
Depending on the variable compositions of terpenes, different cannabis "strains" elicit different aromas, with a greater link to product quality, retail price, and consumer preference.[8][71] The terpene compositions of cannabis are of a seasonal variability. The alteration in the proportion of terpenoids in cannabis are in accordance with the variety of cannabis, plant part, environmental conditions, maturity, and method of analyses.[72][73][74] Different growth stages of cannabis could give considerable variations in the terpene compositions. The terpene profile of cannabis at the vegetative stage is broadly considered to have a much lower proportion of monoterpenes than the flowering stage.[56] Aside from the variations and compositions of terpenes among different phenotypes, the modulated molecular or biological functions of the terpenes are effective only when the concentration of the terpene in the full-spectrum cannabis extract is above 0.05% v/w.[31][68][75]
To characterize the aromatic profile of different chemovars of cannabis, solid phase microextraction (SPME), which is non-destructive and non-invasive, was used to collect the volatiles from the samples.[53][76] This method favors a small sample size and eliminates the use of organic solvents and more importantly, it allows for the emission of hundreds of volatile compounds from the samples.[53][77] Figure 7 shows the chromatograms of the volatile compounds diffusing from the floral tissue of C. sativa var. Northern Light using SPME and gas chromatography. As many as 51 volatiles were detected, in which caryophyllene was dominant, while β-myrcene and limonene were among the major monoterpenes identified.
|
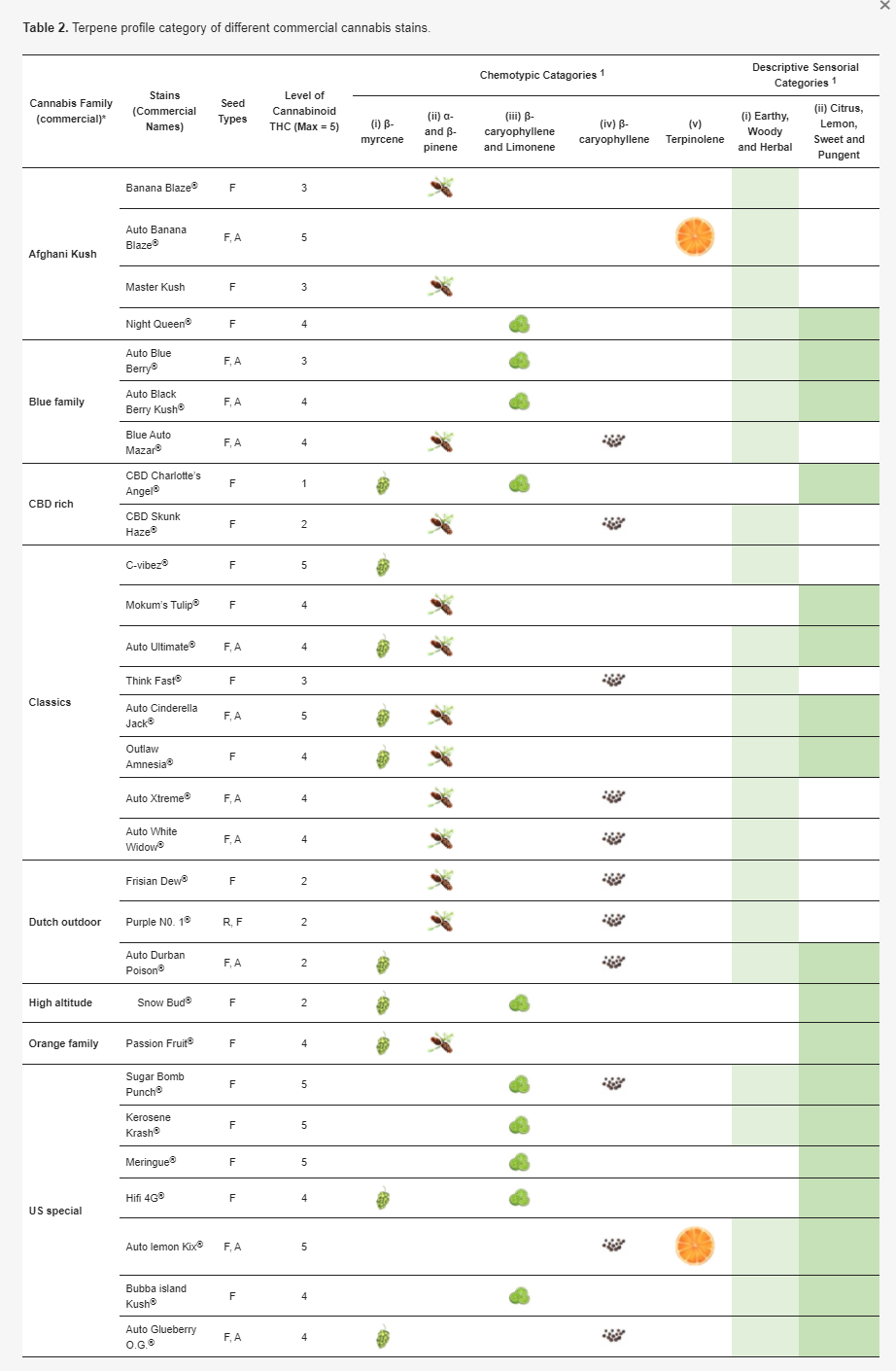
Among the cannabis strains analyzed by Shapira et al.[75], five chemotype groups were elucidated according to the predominant terpenes: (i) β-myrcene, (ii) α- and β-pinene, (iii) β-caryophyllene and limonene, (iv) β-caryophyllene, and (v) terpinolene. In the sensory perception of the terpene profile differences among cannabis strains, two distinct descriptive clustering groups were nominated.[71] The first group included uniformly earthy, woody, and herbal, and the other group comprised the most frequent descriptors, including citrus, lemon, sweet, and pungent. Table 2 shows the lists of cannabis strains available from the Dutch passion seed company (https://dutch-passion.com), classified by the chemotypes and descriptive categories.
Separation of cannabis terpenes and industrial importance
In the past, identifying the terpene profile of cannabis was simply for the purposes of improving canine training aids in illicit drug detection.[76] In the world of the cannabis industry, however, terpenes play a vital role in differentiating the flavor and aroma that are specific to particular strains.[56] Some terpenes can enhance the effect of cannabinoids and synergize the feeling of relaxation, stress relief, energy boost, and maintaining focus, along with all their underlying pharmaceutical functions.[54][79] Thus, a growing number of industries have shown interest in adding either cannabis terpenes or botanically-derived terpenes to their CBD oils and edibles. The estimated growth in this sector should reach a $20 billion market by 2024.[80][81] However, success in this sector might be challenged by a few restraints. First, the consumer believes that the functionality and safety of cannabis products are truly related to sources, perceived novelty, and, most importantly, perceived benefits.[82][83] Additionally, the extraction of full-spectrum oil consisting of a full mix of naturally occurring cannabis terpenes is almost impossible. The most cost-effective way is to selectively separate the terpenes and include them back into the final products.[68][80]
Numerous terpene recovery techniques have been developed, including solvent-based and solvent-free techniques. Essential oils are usually hydro-distillated extracts from the trichomes of cannabis containing mostly terpenes or terpenoids. Although most of the constituents remain intact during distillation, a few monoterpenes may undergo chemical changes or are quite often lost due to the nature of the distillation process.[26][56] The other possible technique is steam distillation, passing dry steam through the inflorescences of the cannabis whereby the terpenes are volatilized, condensed, and collected.[84][85] Moreover, microwave-assisted extraction (MAE) can enrich bioactive compounds. MAE treatment using high irradiation power and relatively long extraction times can significantly increase the content of CBD in the essential oil, with considerably high yield when compared with more conventional hydro-distillation techniques.[86] In Canada, for instance, commercial production of extract is achieved by either solvent extraction using butane or supercritical fluid extraction (SFE), with the restriction on product purity of no solvent contaminants.[87] The latter technique is known to give superior performance in terpene recovery.[88] SFE has recently become a more preferred method for terpene recovery, largely because it allows using lower temperatures, leading to less deterioration of the thermally labile components and is free from organic solvents.[88][89] A supercritical fluid is a fluid at a temperature and pressure above its critical points, with no boundary between the liquid and gas stage. At these points, the fluid is low in viscosity with high diffusion properties to dissolve chemical molecules from the plant matrix. Supercritical carbon dioxide (sCO2) is generally used because it is nonflammable, relatively inexpensive, and non-toxic. Large amounts of terpene ingredients were recovered from this method (i.e., up to 50%, 20%, and 10% of caryophyllene, humulene, and limonene, respectively, can be recovered compared to the conventional methods).[64][89]
Conclusions
Recreational cannabis as a food ingredient, or in other matrices, has become more acceptable in a broader public context where cannabis terpenes have gained high industrial attention in recent years. Terpene profiles not only embody the characteristics of cannabis genotypes, but their entourage effect with cannabinoids could enhance their medicinal functionality. This review highlights the importance of understanding cannabis terpene chemistry and provides descriptive profile categories of different cannabis commercial strains.
Acknowledgements
We would like to especially thank Katsuhisa Komiya for his extended knowledge on the topic. Michael D. Burgett is highly appreciated for his thoughtful language editing of the entire manuscript.
Author contributions
Conceptualization, S.R.S.; Validation, S.R.S. and P.J.; Resources, S.R.S.; Data curation, S.R.S.; Writing—Original draft preparation, S.R.S.; Writing—Review and editing, C.C., W.R., and P.J., Visualization, S.R.S. and P.J.; Funding acquisition, S.R.S. and W.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was partially supported by Chiang Mai University.
Conflicts of interests
The authors declare no conflict of interest.
References
- ↑ Chaohua, Cheng; Gonggu, Zang; Lining, Zhao; Chunsheng, Gao; Qing, Tang; Jianhua, Chen; Xinbo, Guo; Dingxiang, Peng et al. (1 May 2016). "A rapid shoot regeneration protocol from the cotyledons of hemp (Cannabis sativa L.)" (in en). Industrial Crops and Products 83: 61–65. doi:10.1016/j.indcrop.2015.12.035. https://linkinghub.elsevier.com/retrieve/pii/S0926669015306245.
- ↑ Weinberger, Andrea H.; Zhu, Jiaqi; Levin, Jacob; Barrington-Trimis, Jessica L.; Copeland, Jan; Wyka, Katarzyna; Kim, June H.; Goodwin, Renee D. (1 September 2020). "Cannabis use among US adults with anxiety from 2008 to 2017: The role of state-level cannabis legalization". Drug and Alcohol Dependence 214: 108163. doi:10.1016/j.drugalcdep.2020.108163. ISSN 1879-0046. PMID 32707516. https://pubmed.ncbi.nlm.nih.gov/32707516.
- ↑ Rabiee, Rynaz; Lundin, Andreas; Agardh, Emilie; Hensing, Gunnel; Allebeck, Peter; Danielsson, Anna-Karin (1 November 2020). "Cannabis use and the risk of anxiety and depression in women: A comparison of three Swedish cohorts". Drug and Alcohol Dependence 216: 108332. doi:10.1016/j.drugalcdep.2020.108332. ISSN 1879-0046. PMID 33080503. https://pubmed.ncbi.nlm.nih.gov/33080503.
- ↑ Lloyd, Shawnta L.; Lopez-Quintero, Catalina; Striley, Catherine W. (1 November 2020). "Sex differences in driving under the influence of cannabis: The role of medical and recreational cannabis use". Addictive Behaviors 110: 106525. doi:10.1016/j.addbeh.2020.106525. ISSN 1873-6327. PMC 7443537. PMID 32711286. https://pubmed.ncbi.nlm.nih.gov/32711286.
- ↑ Turna, Jasmine; Balodis, Iris; Munn, Catharine; Van Ameringen, Michael; Busse, Jason; MacKillop, James (1 October 2020). "Overlapping patterns of recreational and medical cannabis use in a large community sample of cannabis users". Comprehensive Psychiatry 102: 152188. doi:10.1016/j.comppsych.2020.152188. ISSN 1532-8384. PMID 32653594. https://pubmed.ncbi.nlm.nih.gov/32653594.
- ↑ Theparat, C. (29 January 2020). "New rule makes it legal to grow hemp". Bangkok Post. https://www.bangkokpost.com/thailand/general/1845714/new-rule-makes-it-legal-to-grow-hemp. Retrieved 02 October 2020.
- ↑ 7.0 7.1 Koltai, Hinanit; Namdar, Dvora (1 October 2020). "Cannabis Phytomolecule 'Entourage': From Domestication to Medical Use". Trends in Plant Science 25 (10): 976–984. doi:10.1016/j.tplants.2020.04.007. ISSN 1878-4372. PMID 32417167. https://pubmed.ncbi.nlm.nih.gov/32417167.
- ↑ 8.0 8.1 8.2 Booth, Judith K.; Bohlmann, Jörg (1 July 2019). "Terpenes in Cannabis sativa – From plant genome to humans" (in en). Plant Science 284: 67–72. doi:10.1016/j.plantsci.2019.03.022. https://linkinghub.elsevier.com/retrieve/pii/S0168945219301190.
- ↑ Clarke, R.; Merlin M., ed. (1 September 2013). "History of Cannabis Use for Fiber". Cannabis: Evolution and Ethnobotany. University of California Press. doi:10.1525/9780520954571-011. ISBN 978-0-520-95457-1. https://california.degruyter.com/view/title/554936.
- ↑ Fleming, M.P.; Clarke R.C. (1998). "Physical evidence for the antiquity of Cannabis sativa L.". Journal of the International Hemp Association 5 (2): 80–92. http://www.internationalhempassociation.org/jiha/jiha5208.html.
- ↑ 11.0 11.1 Li, Hui-Lin (1 October 1973). "An archaeological and historical account of cannabis in China" (in en). Economic Botany 28 (4): 437–448. doi:10.1007/BF02862859. ISSN 0013-0001. http://link.springer.com/10.1007/BF02862859.
- ↑ Li, Hui-Lin (1 July 1974). "The origin and use of cannabis in eastern asia linguistic-cultural implications" (in en). Economic Botany 28 (3): 293–301. doi:10.1007/BF02861426. ISSN 0013-0001. http://link.springer.com/10.1007/BF02861426.
- ↑ Hillig, Karl W (1 October 2004). "A chemotaxonomic analysis of terpenoid variation in Cannabis" (in en). Biochemical Systematics and Ecology 32 (10): 875–891. doi:10.1016/j.bse.2004.04.004. https://linkinghub.elsevier.com/retrieve/pii/S0305197804001012.
- ↑ Aluko, R.E. (2017), "Hemp Seed (Cannabis sativa L.) Proteins" (in en), Sustainable Protein Sources (Elsevier): 121–132, doi:10.1016/b978-0-12-802778-3.00007-x, ISBN 978-0-12-802778-3, https://linkinghub.elsevier.com/retrieve/pii/B978012802778300007X
- ↑ Abel, Ernest L. (1980), "Cannabis in the Ancient World" (in en), Marihuana (Boston, MA: Springer US): 3–35, doi:10.1007/978-1-4899-2189-5_1, ISBN 978-1-4899-2191-8, http://link.springer.com/10.1007/978-1-4899-2189-5_1
- ↑ Bonini, Sara Anna; Premoli, Marika; Tambaro, Simone; Kumar, Amit; Maccarinelli, Giuseppina; Memo, Maurizio; Mastinu, Andrea (5 December 2018). "Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history". Journal of Ethnopharmacology 227: 300–315. doi:10.1016/j.jep.2018.09.004. ISSN 1872-7573. PMID 30205181. https://pubmed.ncbi.nlm.nih.gov/30205181.
- ↑ Mediavilla, V; Leupin, M; Keller, A (1 January 2001). "Influence of the growth stage of industrial hemp on the yield formation in relation to certain fibre quality traits" (in en). Industrial Crops and Products 13 (1): 49–56. doi:10.1016/S0926-6690(00)00052-2. https://linkinghub.elsevier.com/retrieve/pii/S0926669000000522.
- ↑ Cosentino, Salvatore L.; Riggi, Ezio; Testa, Giorgio; Scordia, Danilo; Copani, Venera (1 October 2013). "Evaluation of European developed fibre hemp genotypes (Cannabis sativa L.) in semi-arid Mediterranean environment" (in en). Industrial Crops and Products 50: 312–324. doi:10.1016/j.indcrop.2013.07.059. https://linkinghub.elsevier.com/retrieve/pii/S0926669013003968.
- ↑ 19.0 19.1 Bewley-Taylor, D.; Blickman T.; Jelsma, M. (March 2014). "The Rise and Decline of Cannabis Prohibition: The History of Cannabis in the UN Drug Control System and Options for Reform". In Aronson, D. (PDF). Transnational Institute. https://www.tni.org/files/download/rise_and_decline_web.pdf.
- ↑ Gaoni, Y.; Mechoulam, R. (1 April 1964). "Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish" (in en). Journal of the American Chemical Society 86 (8): 1646–1647. doi:10.1021/ja01062a046. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja01062a046.
- ↑ Šantavý, F. (1964). "Notes on the structure of cannabidiol compounds". Acta Universitatis Palackianae Olomucensis Facultatis Medicae 35: 5–9.
- ↑ Adams, Roger; Pease, D. C.; Cain, C. K.; Baker, B. R.; Clark, J. H.; Wolff, Hans; Wearn, R. B. (1 August 1940). "CONVERSION OF CANNABIDIOL TO A PRODUCT WITH MARIHUANA ACTIVITY. A TYPE REACTION FOR SYNTHESIS OF ANALOGOUS SUBSTANCES. CONVERSION OF CANNABIDIOL TO CANNABINOL" (in en). Journal of the American Chemical Society 62 (8): 2245–2246. doi:10.1021/ja01865a508. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja01865a508.
- ↑ Devane, W. A.; Dysarz, F. A.; Johnson, M. R.; Melvin, L. S.; Howlett, A. C. (1 November 1988). "Determination and characterization of a cannabinoid receptor in rat brain". Molecular Pharmacology 34 (5): 605–613. ISSN 0026-895X. PMID 2848184. https://pubmed.ncbi.nlm.nih.gov/2848184.
- ↑ Devane, William A.; Hanuš, Lumir; Breuer, Aviva; Pertwee, Roger G.; Stevenson, Lesley A.; Griffin, Graeme; Gibson, Dan; Mandelbaum, Asher et al. (18 December 1992). "Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor" (in en). Science 258 (5090): 1946–1949. doi:10.1126/science.1470919. ISSN 0036-8075. https://www.science.org/doi/10.1126/science.1470919.
- ↑ Simonsen, J. L.; Todd, A. R. (1942). "32. Cannabis indica. Part X. The essential oil from Egyptian hashish" (in en). Journal of the Chemical Society (Resumed): 188. doi:10.1039/jr9420000188. ISSN 0368-1769. http://xlink.rsc.org/?DOI=jr9420000188.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 Hanuš, Lumír Ondřej; Hod, Yotam (10 August 2020). "Terpenes/Terpenoids in Cannabis: Are They Important?" (in en). Medical Cannabis and Cannabinoids 3 (1): 25–60. doi:10.1159/000509733. ISSN 2504-3889. PMC PMC8489319. PMID 34676339. https://www.karger.com/Article/FullText/509733.
- ↑ Ben-Shabat, Shimon; Fride, Ester; Sheskin, Tzviel; Tamiri, Tsippy; Rhee, Man-Hee; Vogel, Zvi; Bisogno, Tiziana; De Petrocellis, Luciano et al. (1 July 1998). "An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity" (in en). European Journal of Pharmacology 353 (1): 23–31. doi:10.1016/S0014-2999(98)00392-6. https://linkinghub.elsevier.com/retrieve/pii/S0014299998003926.
- ↑ 28.0 28.1 28.2 28.3 Russo, Ethan B (1 August 2011). "Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects: Phytocannabinoid-terpenoid entourage effects" (in en). British Journal of Pharmacology 163 (7): 1344–1364. doi:10.1111/j.1476-5381.2011.01238.x. PMC PMC3165946. PMID 21749363. https://onlinelibrary.wiley.com/doi/10.1111/j.1476-5381.2011.01238.x.
- ↑ Gallily, Ruth; Yekhtin, Zhannah; Hanuš, Lumír Ondřej (2018). "The Anti-Inflammatory Properties of Terpenoids from Cannabis". Cannabis and Cannabinoid Research 3 (1): 282–290. doi:10.1089/can.2018.0014. ISSN 2578-5125. PMC 6308289. PMID 30596146. https://pubmed.ncbi.nlm.nih.gov/30596146.
- ↑ Baron, Eric P. (1 July 2018). "Medicinal Properties of Cannabinoids, Terpenes, and Flavonoids in Cannabis, and Benefits in Migraine, Headache, and Pain: An Update on Current Evidence and Cannabis Science". Headache 58 (7): 1139–1186. doi:10.1111/head.13345. ISSN 1526-4610. PMID 30152161. https://pubmed.ncbi.nlm.nih.gov/30152161.
- ↑ 31.0 31.1 Lewis, Mark A.; Russo, Ethan B.; Smith, Kevin M. (1 March 2018). "Pharmacological Foundations of Cannabis Chemovars". Planta Medica 84 (4): 225–233. doi:10.1055/s-0043-122240. ISSN 1439-0221. PMID 29161743. https://pubmed.ncbi.nlm.nih.gov/29161743.
- ↑ Horne, Matthew R.L. (2020), "Bast fibres" (in en), Handbook of Natural Fibres (Elsevier): 163–196, doi:10.1016/b978-0-12-818398-4.00007-4, ISBN 978-0-12-818398-4, https://linkinghub.elsevier.com/retrieve/pii/B9780128183984000074
- ↑ Réquilé, Samuel; Le Duigou, Antoine; Bourmaud, Alain; Baley, Christophe (1 November 2018). "Peeling experiments for hemp retting characterization targeting biocomposites" (in en). Industrial Crops and Products 123: 573–580. doi:10.1016/j.indcrop.2018.07.012. https://linkinghub.elsevier.com/retrieve/pii/S0926669018306125.
- ↑ Anderson, Loran C. (1 March 1980). "Leaf Variation among Cannabis Species from a Controlled Garden". Botanical Museum leaflets, Harvard University 28 (1): 61–69. doi:10.5962/p.168641. ISSN 0006-8098. https://www.biodiversitylibrary.org/part/168641.
- ↑ Schultes, Richard Evans; Klein, William M.; Plowman, Timothy; Lockwood, Tom E. (31 December 1975), Rubin, Vera, ed., "Cannabis: An Example of Taxonomic Neglect", Cannabis and Culture (DE GRUYTER MOUTON): 21–38, doi:10.1515/9783110812060.21, ISBN 978-90-279-7669-7, https://www.degruyter.com/document/doi/10.1515/9783110812060.21/html
- ↑ 36.0 36.1 Cai, Changyong; Yu, Wang; Wang, Chaoyun; Liu, Lianglei; Li, Fenfang; Tan, Zhijian (1 August 2019). "Green extraction of cannabidiol from industrial hemp (Cannabis sativa L.) using deep eutectic solvents coupled with further enrichment and recovery by macroporous resin" (in en). Journal of Molecular Liquids 287: 110957. doi:10.1016/j.molliq.2019.110957. https://linkinghub.elsevier.com/retrieve/pii/S0167732218365899.
- ↑ Wiebelhaus, Nancy; Hamblin, D’Nisha; Kreitals, Natasha M.; Almirall, Jose R. (1 November 2016). "Differentiation of marijuana headspace volatiles from other plants and hemp products using capillary microextraction of volatiles (CMV) coupled to gas-chromatography–mass spectrometry (GC–MS)" (in en). Forensic Chemistry 2: 1–8. doi:10.1016/j.forc.2016.08.004. https://linkinghub.elsevier.com/retrieve/pii/S2468170916300285.
- ↑ Hammond, Charles T.; Mahlberg, Paul G. (1 September 1977). "MORPHOGENESIS OF CAPITATE GLANDULAR HAIRS OF CANNABIS SATIVA (CANNABACEAE)" (in en). American Journal of Botany 64 (8): 1023–1031. doi:10.1002/j.1537-2197.1977.tb11948.x. https://onlinelibrary.wiley.com/doi/10.1002/j.1537-2197.1977.tb11948.x.
- ↑ Potter, D. (March 2009). "The Propagation, Characterisation and Optimisation of Cannabis sativa L. as a Phytopharmaceutical" (PDF). King’s College London. https://extractionmagazine.com/wp-content/uploads/2018/06/THE-PROPAGATION-CHARACTERISATION-AND-OPTIMISATION-OF-CANNABIS-SATIVA-L-AS-A-PHYTOPHARMACEUTICAL.pdf.
- ↑ 40.0 40.1 Livingston, Samuel J.; Quilichini, Teagen D.; Booth, Judith K.; Wong, Darren C. J.; Rensing, Kim H.; Laflamme-Yonkman, Jessica; Castellarin, Simone D.; Bohlmann, Joerg et al. (1 January 2020). "Cannabis glandular trichomes alter morphology and metabolite content during flower maturation". The Plant Journal: For Cell and Molecular Biology 101 (1): 37–56. doi:10.1111/tpj.14516. ISSN 1365-313X. PMID 31469934. https://pubmed.ncbi.nlm.nih.gov/31469934.
- ↑ Gupta, Asheesh Kumar; Jain, Anurekha; Roy, Partha; Singh, Ramandeep (2 October 2020). "Pharmacological Evaluation of Cannabis indica For Their Aphrodisiac Potential". International Journal of Ayurvedic Medicine 11 (3): 399–404. doi:10.47552/ijam.v11i3.1647. ISSN 0976-5921. https://www.ijam.co.in/index.php/ijam/article/view/1647.
- ↑ Ahmed, Aziez; Shapiro, Douglas; Su, Jennifer; Nelson, Lara P. (1 May 2021). "Vaping Cannabis Butane Hash Oil Leads to Severe Acute Respiratory Distress Syndrome-A Case of EVALI in a Teenager With Hypertrophic Cardiomyopathy". Journal of Intensive Care Medicine 36 (5): 617–621. doi:10.1177/0885066620941004. ISSN 1525-1489. PMID 32686568. https://pubmed.ncbi.nlm.nih.gov/32686568.
- ↑ Stephens, Danica; Patel, Jinal K.; Angelo, Debra; Frunzi, Johnathan (18 February 2020). "Cannabis Butane Hash Oil Dabbing Induced Lung Injury Mimicking Atypical Pneumonia". Cureus 12 (2): e7033. doi:10.7759/cureus.7033. ISSN 2168-8184. PMC 7082782. PMID 32211266. https://pubmed.ncbi.nlm.nih.gov/32211266.
- ↑ Schwachtje, Jens; Baldwin, Ian T. (1 March 2008). "Why does herbivore attack reconfigure primary metabolism?". Plant Physiology 146 (3): 845–851. doi:10.1104/pp.107.112490. ISSN 0032-0889. PMC 2259057. PMID 18316639. https://pubmed.ncbi.nlm.nih.gov/18316639.
- ↑ Sommano, Sarana (30 November 2013), P. M, Visakh; Iturriaga, Laura B.; Ribotta, Pablo Daniel, eds., "Effect of Food Processing on Bioactive Compounds" (in en), Advances in Food Science and Nutrition (Hoboken, NJ, USA: John Wiley & Sons, Inc.): 361–390, doi:10.1002/9781118865606.ch11, ISBN 978-1-118-86560-6, https://onlinelibrary.wiley.com/doi/10.1002/9781118865606.ch11
- ↑ Sommano, Sarana; Caffin, Nola; Kerven, Graham (18 August 2013). "Screening for Antioxidant Activity, Phenolic Content, and Flavonoids from Australian Native Food Plants" (in en). International Journal of Food Properties 16 (6): 1394–1406. doi:10.1080/10942912.2011.580485. ISSN 1094-2912. http://www.tandfonline.com/doi/abs/10.1080/10942912.2011.580485.
- ↑ Sunanta, Piyachat; Chung, Hsiao‐Hang; Kunasakdakul, Kaewalin; Ruksiriwanich, Warintorn; Jantrawut, Pensak; Hongsibsong, Surat; Sommano, Sarana Rose (1 August 2020). "Genomic relationship and physiochemical properties among raw materials used for Thai black garlic processing" (in en). Food Science & Nutrition 8 (8): 4534–4545. doi:10.1002/fsn3.1762. ISSN 2048-7177. PMC PMC7455981. PMID 32884733. https://onlinelibrary.wiley.com/doi/10.1002/fsn3.1762.
- ↑ Wisetkomolmat, Jiratchaya; Suppakittpaisarn, Pongsakorn; Sommano, Sarana Rose (3 January 2019). "Detergent Plants of Northern Thailand: Potential Sources of Natural Saponins" (in en). Resources 8 (1): 10. doi:10.3390/resources8010010. ISSN 2079-9276. https://www.mdpi.com/2079-9276/8/1/10.
- ↑ 49.0 49.1 Tangpao, Tibet; Chung, Hsiao-Hang; Sommano, Sarana (24 October 2018). "Aromatic Profiles of Essential Oils from Five Commonly Used Thai Basils" (in en). Foods 7 (11): 175. doi:10.3390/foods7110175. ISSN 2304-8158. PMC PMC6262289. PMID 30352978. http://www.mdpi.com/2304-8158/7/11/175.
- ↑ Ross, Samir A.; ElSohly, Mahmoud A. (1 January 1996). "The Volatile Oil Composition of Fresh and Air-Dried Buds of Cannabis sativa" (in en). Journal of Natural Products 59 (1): 49–51. doi:10.1021/np960004a. ISSN 0163-3864. https://pubs.acs.org/doi/10.1021/np960004a.
- ↑ Turner, C. E.; Elsohly, M. A.; Boeren, E. G. (1 March 1980). "Constituents of Cannabis sativa L. XVII. A review of the natural constituents". Journal of Natural Products 43 (2): 169–234. doi:10.1021/np50008a001. ISSN 0163-3864. PMID 6991645. https://pubmed.ncbi.nlm.nih.gov/6991645.
- ↑ 52.0 52.1 Wanas, Amira S.; Radwan, Mohamed M.; Chandra, Suman; Lata, Hemant; Mehmedic, Zlatko; Ali, Abbas; Baser, Khc; Demirci, Betul et al. (1 May 2020). "Chemical Composition of Volatile Oils of Fresh and Air-Dried Buds of Cannabis c hemovars, Their Insecticidal and Repellent Activities" (in en). Natural Product Communications 15 (5): 1934578X2092672. doi:10.1177/1934578X20926729. ISSN 1934-578X. http://journals.sagepub.com/doi/10.1177/1934578X20926729.
- ↑ 53.0 53.1 53.2 Rice, Somchai; Koziel, Jacek A. (10 December 2015). Glendinning, John I.. ed. "Characterizing the Smell of Marijuana by Odor Impact of Volatile Compounds: An Application of Simultaneous Chemical and Sensory Analysis" (in en). PLOS ONE 10 (12): e0144160. doi:10.1371/journal.pone.0144160. ISSN 1932-6203. PMC PMC4684335. PMID 26657499. https://dx.plos.org/10.1371/journal.pone.0144160.
- ↑ 54.0 54.1 Ternelli, Marco; Brighenti, Virginia; Anceschi, Lisa; Poto, Massimiliano; Bertelli, Davide; Licata, Manuela; Pellati, Federica (15 July 2020). "Innovative methods for the preparation of medical Cannabis oils with a high content of both cannabinoids and terpenes". Journal of Pharmaceutical and Biomedical Analysis 186: 113296. doi:10.1016/j.jpba.2020.113296. ISSN 1873-264X. PMID 32334134. https://pubmed.ncbi.nlm.nih.gov/32334134.
- ↑ Wang, Chi-Tsan; Ashworth, Kirsti; Wiedinmyer, Christine; Ortega, John; Harley, Peter C.; Rasool, Quazi Z.; Vizuete, William (1 July 2020). "Ambient measurements of monoterpenes near Cannabis cultivation facilities in Denver, Colorado" (in en). Atmospheric Environment 232: 117510. doi:10.1016/j.atmosenv.2020.117510. https://linkinghub.elsevier.com/retrieve/pii/S1352231020302478.
- ↑ 56.0 56.1 56.2 56.3 Abdollahi, Mahnaz; Sefidkon, Fatemeh; Calagari, Mohsen; Mousavi, Amir; Mahomoodally, M. Fawzi (1 November 2020). "Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils" (in en). Industrial Crops and Products 155: 112793. doi:10.1016/j.indcrop.2020.112793. https://linkinghub.elsevier.com/retrieve/pii/S092666902030710X.
- ↑ Sriwichai, Trid; Junmahasathien, Taepin; Sookwong, Phumon; Potapohn, Nuttha; Sommano, Sarana Rose (17 April 2019). "Evaluation of the Optimum Harvesting Maturity of Makhwaen Fruit for the Perfumery Industry" (in en). Agriculture 9 (4): 78. doi:10.3390/agriculture9040078. ISSN 2077-0472. https://www.mdpi.com/2077-0472/9/4/78.
- ↑ 58.0 58.1 Booth, Judith K.; Page, Jonathan E.; Bohlmann, Jörg (29 March 2017). Hamberger, Björn. ed. "Terpene synthases from Cannabis sativa" (in en). PLOS ONE 12 (3): e0173911. doi:10.1371/journal.pone.0173911. ISSN 1932-6203. PMC PMC5371325. PMID 28355238. https://dx.plos.org/10.1371/journal.pone.0173911.
- ↑ Nagegowda, Dinesh A.; Gupta, Priyanka (1 May 2020). "Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids" (in en). Plant Science 294: 110457. doi:10.1016/j.plantsci.2020.110457. https://linkinghub.elsevier.com/retrieve/pii/S0168945220300595.
- ↑ Nagegowda, Dinesh A.; Gupta, Priyanka (1 May 2020). "Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids" (in en). Plant Science 294: 110457. doi:10.1016/j.plantsci.2020.110457. https://linkinghub.elsevier.com/retrieve/pii/S0168945220300595.
- ↑ 61.0 61.1 Fellermeier, M.; Eisenreich, W.; Bacher, A.; Zenk, M. H. (1 March 2001). "Biosynthesis of cannabinoids. Incorporation experiments with (13)C-labeled glucoses". European Journal of Biochemistry 268 (6): 1596–1604. doi:10.1046/j.1432-1033.2001.02030.x. ISSN 0014-2956. PMID 11248677. https://pubmed.ncbi.nlm.nih.gov/11248677.
- ↑ Aliferis, Konstantinos A.; Bernard-Perron, David (2020). "Cannabinomics: Application of Metabolomics in Cannabis (Cannabis sativa L.) Research and Development". Frontiers in Plant Science 11: 554. doi:10.3389/fpls.2020.00554. ISSN 1664-462X. PMC 7225349. PMID 32457786. https://pubmed.ncbi.nlm.nih.gov/32457786.
- ↑ Masoud, Asaad N.; Doorenbos, Norman J. (1 February 1973). "Mississippi-Grown Cannabis sativa L. III: Cannabinoid and Cannabinoid Acid Content" (in en). Journal of Pharmaceutical Sciences 62 (2): 313–315. doi:10.1002/jps.2600620229. https://linkinghub.elsevier.com/retrieve/pii/S0022354915388912.
- ↑ 64.0 64.1 Grijó, Daniel Ribeiro; Vieitez Osorio, Ignacio Alberto; Cardozo-Filho, Lúcio (1 December 2018). "Supercritical extraction strategies using CO2 and ethanol to obtain cannabinoid compounds from Cannabis hybrid flowers" (in en). Journal of CO2 Utilization 28: 174–180. doi:10.1016/j.jcou.2018.09.022. https://linkinghub.elsevier.com/retrieve/pii/S2212982018304220.
- ↑ Miyazawa, Mitsuo; Yamafuji, Chikako (9 March 2005). "Inhibition of acetylcholinesterase activity by bicyclic monoterpenoids". Journal of Agricultural and Food Chemistry 53 (5): 1765–1768. doi:10.1021/jf040019b. ISSN 0021-8561. PMID 15740071. https://pubmed.ncbi.nlm.nih.gov/15740071.
- ↑ 66.0 66.1 66.2 66.3 Gaggiotti, Sara; Palmieri, Sara; Della Pelle, Flavio; Sergi, Manuel; Cichelli, Angelo; Mascini, Marcello; Compagnone, Dario (1 April 2020). "Piezoelectric peptide-hpDNA based electronic nose for the detection of terpenes; Evaluation of the aroma profile in different Cannabis sativa L. (hemp) samples" (in en). Sensors and Actuators B: Chemical 308: 127697. doi:10.1016/j.snb.2020.127697. https://linkinghub.elsevier.com/retrieve/pii/S0925400520300447.
- ↑ Sriwichai, Trid; Sookwong, Phumon; Siddiqui, Mohammed Wasim; Sommano, Sarana Rose (1 July 2019). "Aromatic profiling of Zanthoxylum myriacanthum (makwhaen) essential oils from dried fruits using different initial drying techniques" (in en). Industrial Crops and Products 133: 284–291. doi:10.1016/j.indcrop.2019.03.031. https://linkinghub.elsevier.com/retrieve/pii/S0926669019301888.
- ↑ 68.0 68.1 68.2 68.3 Maayah, Zaid H.; Takahara, Shingo; Ferdaoussi, Mourad; Dyck, Jason R.B. (1 July 2020). "The molecular mechanisms that underpin the biological benefits of full-spectrum cannabis extract in the treatment of neuropathic pain and inflammation" (in en). Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1866 (7): 165771. doi:10.1016/j.bbadis.2020.165771. https://linkinghub.elsevier.com/retrieve/pii/S0925443920301162.
- ↑ Rao, V S N; Menezes, A M S; Viana, G S B (12 April 2011). "Effect of myrcene on nociception in mice" (in en). Journal of Pharmacy and Pharmacology 42 (12): 877–878. doi:10.1111/j.2042-7158.1990.tb07046.x. ISSN 2042-7158. https://academic.oup.com/jpp/article/42/12/877/6164538.
- ↑ Meschler, J (1 March 1999). "Thujone Exhibits Low Affinity for Cannabinoid Receptors But Fails to Evoke Cannabimimetic Responses". Pharmacology Biochemistry and Behavior 62 (3): 473–480. doi:10.1016/S0091-3057(98)00195-6. https://linkinghub.elsevier.com/retrieve/pii/S0091305798001956.
- ↑ 71.0 71.1 Gilbert, Avery N.; DiVerdi, Joseph A. (2018). "Consumer perceptions of strain differences in Cannabis aroma". PloS One 13 (2): e0192247. doi:10.1371/journal.pone.0192247. ISSN 1932-6203. PMC 5798829. PMID 29401526. https://pubmed.ncbi.nlm.nih.gov/29401526.
- ↑ Brenneisen, Rudolf (2007), ElSohly, Mahmoud A., ed., "Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents" (in en), Marijuana and the Cannabinoids (Totowa, NJ: Humana Press): 17–49, doi:10.1007/978-1-59259-947-9_2, ISBN 978-1-58829-456-2, http://link.springer.com/10.1007/978-1-59259-947-9_2
- ↑ Fischedick, Justin Thomas; Hazekamp, Arno; Erkelens, Tjalling; Choi, Young Hae; Verpoorte, Rob (1 December 2010). "Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes". Phytochemistry 71 (17-18): 2058–2073. doi:10.1016/j.phytochem.2010.10.001. ISSN 1873-3700. PMID 21040939. https://pubmed.ncbi.nlm.nih.gov/21040939.
- ↑ Brown, Alistair K.; Xia, Zhe; Bulloch, Patrique; Idowu, Ifeoluwa; Francisco, Olga; Stetefeld, Jorg; Stout, Jake; Zimmer, Jeff et al. (11 December 2019). "Validated quantitative cannabis profiling for Canadian regulatory compliance - Cannabinoids, aflatoxins, and terpenes". Analytica Chimica Acta 1088: 79–88. doi:10.1016/j.aca.2019.08.042. ISSN 1873-4324. PMID 31623719. https://pubmed.ncbi.nlm.nih.gov/31623719.
- ↑ 75.0 75.1 75.2 Shapira, Anna; Berman, Paula; Futoran, Kate; Guberman, Ohad; Meiri, David (3 September 2019). "Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections" (in en). Analytical Chemistry 91 (17): 11425–11432. doi:10.1021/acs.analchem.9b02844. ISSN 0003-2700. https://pubs.acs.org/doi/10.1021/acs.analchem.9b02844.
- ↑ 76.0 76.1 Rice, Somchai; Koziel, Jacek A. (1 December 2015). "The relationship between chemical concentration and odor activity value explains the inconsistency in making a comprehensive surrogate scent training tool representative of illicit drugs" (in en). Forensic Science International 257: 257–270. doi:10.1016/j.forsciint.2015.08.027. https://linkinghub.elsevier.com/retrieve/pii/S0379073815003710.
- ↑ Kabir, Abuzar; Holness, Howard; Furton, Kenneth G.; Almirall, José R. (1 April 2013). "Recent advances in micro-sample preparation with forensic applications" (in en). TrAC Trends in Analytical Chemistry 45: 264–279. doi:10.1016/j.trac.2012.11.013. https://linkinghub.elsevier.com/retrieve/pii/S0165993613000241.
- ↑ Calvi, Lorenzo; Pentimalli, Daniela; Panseri, Sara; Giupponi, Luca; Gelmini, Fabrizio; Beretta, Giangiacomo; Vitali, Davide; Bruno, Massimo et al. (1 February 2018). "Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach" (in en). Journal of Pharmaceutical and Biomedical Analysis 150: 208–219. doi:10.1016/j.jpba.2017.11.073. https://linkinghub.elsevier.com/retrieve/pii/S0731708517325086.
- ↑ Koltai, Hinanit; Poulin, Patrick; Namdar, Dvory (1 April 2019). "Promoting cannabis products to pharmaceutical drugs" (in en). European Journal of Pharmaceutical Sciences 132: 118–120. doi:10.1016/j.ejps.2019.02.027. https://linkinghub.elsevier.com/retrieve/pii/S0928098719300880.
- ↑ 80.0 80.1 Koby M. (20 April 2020). "How Terpenes Could Revolutionize The Cannabis Industry As We Know It". Green Entrepreneur. https://www.greenentrepreneur.com/article/349207.
- ↑ King, Jerry W (1 August 2019). "The relationship between cannabis/hemp use in foods and processing methodology" (in en). Current Opinion in Food Science 28: 32–40. doi:10.1016/j.cofs.2019.04.007. https://linkinghub.elsevier.com/retrieve/pii/S2214799319300244.
- ↑ Charlebois, Sylvain; Somogyi, Simon; Sterling, Brian (1 April 2018). "Cannabis-infused food and Canadian consumers’ willingness to consider “recreational” cannabis as a food ingredient" (in en). Trends in Food Science & Technology 74: 112–118. doi:10.1016/j.tifs.2018.02.009. https://linkinghub.elsevier.com/retrieve/pii/S0924224417306209.
- ↑ Khan, Rao Sanaullah; Grigor, John; Winger, Ray; Win, Alan (1 March 2013). "Functional food product development – Opportunities and challenges for food manufacturers" (in en). Trends in Food Science & Technology 30 (1): 27–37. doi:10.1016/j.tifs.2012.11.004. https://linkinghub.elsevier.com/retrieve/pii/S0924224412002592.
- ↑ Benelli, Giovanni; Pavela, Roman; Petrelli, Riccardo; Cappellacci, Loredana; Santini, Giuseppe; Fiorini, Dennis; Sut, Stefania; Dall’Acqua, Stefano et al. (1 October 2018). "The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops" (in en). Industrial Crops and Products 122: 308–315. doi:10.1016/j.indcrop.2018.05.032. https://linkinghub.elsevier.com/retrieve/pii/S0926669018304515.
- ↑ Hanif, Muhammad Asif; Nawaz, Haq; Naz, Saima; Mukhtar, Rubina; Rashid, Nosheen; Bhatti, Ijaz Ahmad; Saleem, Muhammad (5 July 2017). "Raman spectroscopy for the characterization of different fractions of hemp essential oil extracted at 130°C using steam distillation method". Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy 182: 168–174. doi:10.1016/j.saa.2017.03.072. ISSN 1873-3557. PMID 28431313. https://pubmed.ncbi.nlm.nih.gov/28431313.
- ↑ Fiorini, Dennis; Scortichini, Serena; Bonacucina, Giulia; Greco, Nicolas G.; Mazzara, Eugenia; Petrelli, Riccardo; Torresi, Jacopo; Maggi, Filippo et al. (1 October 2020). "Cannabidiol-enriched hemp essential oil obtained by an optimized microwave-assisted extraction using a central composite design" (in en). Industrial Crops and Products 154: 112688. doi:10.1016/j.indcrop.2020.112688. https://linkinghub.elsevier.com/retrieve/pii/S092666902030604X.
- ↑ Blake, Alexia; Nahtigal, Istok (1 August 2019). "The evolving landscape of cannabis edibles" (in en). Current Opinion in Food Science 28: 25–31. doi:10.1016/j.cofs.2019.03.009. https://linkinghub.elsevier.com/retrieve/pii/S2214799319300141.
- ↑ 88.0 88.1 Baldino, Lucia; Scognamiglio, Mariarosa; Reverchon, Ernesto (1 November 2020). "Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review" (in en). The Journal of Supercritical Fluids 165: 104960. doi:10.1016/j.supflu.2020.104960. https://linkinghub.elsevier.com/retrieve/pii/S0896844620302114.
- ↑ 89.0 89.1 Naz, Saima; Hanif, Muhammad Asif; Bhatti, Haq Nawaz; Ansari, Tariq Mahmood (2 January 2017). "Impact of Supercritical Fluid Extraction and Traditional Distillation on the Isolation of Aromatic Compounds from Cannabis indica and Cannabis sativa". Journal of Essential Oil Bearing Plants 20 (1): 175–184. doi:10.1080/0972060X.2017.1281766. ISSN 0972-060X. https://doi.org/10.1080/0972060X.2017.1281766.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added.