Sigma metrics as a valuable tool for effective analytical performance and quality control planning in the clinical laboratory: A retrospective study
Contents
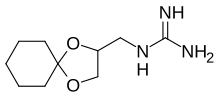
| |
| Names | |
|---|---|
| IUPAC name
2-(1,4-Dioxaspiro[4.5]decan-2-ylmethyl)guanidine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
| MeSH | C004945 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H19N3O2 | |
| Molar mass | 213.281 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Guanadrel is an antihypertensive agent.[1] It is used in the form of its sulfate.
Mechanism of action
Guanadrel is a postganglionic adrenergic blocking agent. Uptake of guanadrel and storage in sympathetic neurons occurs via the norepinephrine pump; guanadrel slowly displaces norepinephrine from its storage in nerve endings and thereby blocks the release of norepinephrine normally produced by nerve stimulation. The reduction in neurotransmitter release in response to sympathetic nerve stimulation, as a result of catecholamine depletion, leads to reduced arteriolar vasoconstriction, especially the reflex increase in sympathetic tone that occurs with a change in position. Guanadrel is rapidly and well absorbed from gastrointestinal tract.[2]
In 1981 the JAMA reported guanadrel as an effective step II or step III treatment of hypertension.[3]
References
- ^ Oren A, Rotmensch HH, Vlasses PH, et al. (1985). "A dose-titration trial of guanadrel as step-two therapy in essential hypertension". J Clin Pharmacol. 25 (5): 343–6. doi:10.1002/j.1552-4604.1985.tb02852.x. PMID 4031111.[permanent dead link]
- ^ Guanadrel, from Pharmacogenetics Knowledge Base
- ^ M. I. Dunn and J. L. Dunlap (1981). "Guanadrel. A new antihypertensive drug". JAMA. 245 (16): 1639–42. doi:10.1001/jama.1981.03310410017019. PMID 7206175.

















