Sigma metrics as a valuable tool for effective analytical performance and quality control planning in the clinical laboratory: A retrospective study
Contents
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H28N2O3 |
| Molar mass | 380.488 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Bopindolol (INN) is a beta blocker. It is an ester which acts as a prodrug for its active metabolite 4-(3-t-butylamino-2-hydroxypropoxy)-2-methylindole.[1]
Synthesis
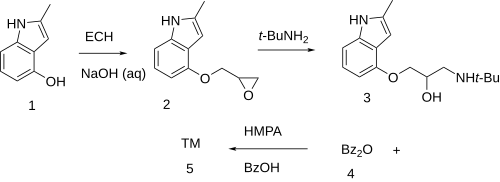
The reaction of 4-Hydroxy-2-methylindole [35320-67-3] (1) with epichlorohydrin in the presence of lye led to 2-methyl-4-(oxiran-2-ylmethoxy)-1H-indole [62119-47-5] (2). Addition of tert-butylamine led to 4-(2-Hydroxy-3-tert-butylaminopropoxy)-2-methylindole [23869-98-9] (3). Ester formation with benzoic anhydride [93-97-0] (4) in the presence of hexamethylphosphoric acid triamide [680-31-9] completed the synthesis of Bopindolol (5).
See also
References
- ^ Nagatomo T, Hosohata Y, Ohnuki T, Nakamura T, Hattori K, Suzuki J, Ishiguro M (Spring 2001). "Bopindolol: pharmacological basis and clinical implications". Cardiovascular Drug Reviews. 19 (1): 9–24. doi:10.1111/j.1527-3466.2001.tb00180.x. PMID 11314603.
- ^ DE2635209 idem Franz Troxler, Fritz Seemann, U.S. patent 4,434,176 (1984 to Sandoz Ltd.).
- ^ Franz Dr Troxler & Albert Dr Hofmann, CH453363 (1968 to Sandoz AG).

















