Risk assessment of over-the-counter cannabinoid-based cosmetics: Legal and regulatory issues governing the safety of cannabinoid-based cosmetics in the UAE
Contents
| |
| Names | |
|---|---|
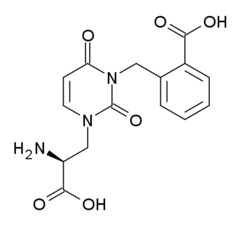
| Systematic IUPAC name
2-({3-[(2S)-2-Amino-2-carboxyethyl]-2,6-dioxo-3,6-dihydropyrimidin-1(2H)-yl}methyl)benzoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.210.061 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H15N3O6 | |
| Molar mass | 333.296 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
UBP-302 is a highly selective kainate receptor antagonist used in the study of many neurological processes. It is active at micromolar concentration within an in vitro preparation and specifically targets the GluK1 (iGluR5) subunit of the receptor. This compound was developed at the University of Bristol.[1]
UBP-310 and UBP-316 (ACET) are related N3-substituted willardiine derivatives.[2]
References
- ^ More, JC; Nistico, R; Dolman, NP; Clarke, VR; Alt, AJ; Ogden, AM; Buelens, FP; Troop, HM; et al. (2004). "Characterisation of UBP296: a novel, potent and selective kainate receptor antagonist". Neuropharmacology. 47 (1): 46–64. doi:10.1016/j.neuropharm.2004.03.005. hdl:11573/514325. PMID 15165833. S2CID 40775500.
- ^ Dolman, NP; More, JCA; Alt, A; Knauss, JL; Pentikäinen, OT; Glasser, CR; Bleakman, D; Mayer, ML; Collingridge, GL; Jane, DE (2007-04-05). "Synthesis and pharmacological characterization of N3-substituted willardiine derivatives: role of the substituent at the 5-position of the uracil ring in the development of highly potent and selective GLUK5 kainate receptor antagonists". Journal of Medicinal Chemistry. 50 (7): 1558–1570. doi:10.1021/jm061041u. ISSN 0022-2623. PMID 17348638.

















