Risk assessment of over-the-counter cannabinoid-based cosmetics: Legal and regulatory issues governing the safety of cannabinoid-based cosmetics in the UAE
Contents
OlliverWithDoubleL (talk | contribs) added chem formatting Tags: Mobile edit Mobile app edit iOS app edit |
CommonsDelinker (talk | contribs) Replacing S-butyl_acetate.svg with File:Sec-butyl_acetate.svg (by CommonsDelinker because: File renamed: "S" has a specific and different meaning that could be relevant to this chemical but is not for thi |
||
| (36 intermediate revisions by 11 users not shown) | |||
| Line 1: | Line 1: | ||
{{Short description| |
{{Short description|Chemical group (−C₄H₉) derived from butane}} |
||
{{Redirect|Butyl|the synthetic rubber|Butyl rubber}} |
{{Redirect|Butyl|the synthetic rubber|Butyl rubber}} |
||
In [[organic chemistry]], '''butyl''' is a four-[[carbon]] [[alkyl]] [[radical (chemistry)|radical]] or [[ |
In [[organic chemistry]], '''butyl''' is a four-[[carbon]] [[alkyl]] [[radical (chemistry)|radical]] or [[Substituent|substituent group]] with general [[chemical formula]] {{chem2|\sC4H9}}, derived from either of the two [[isomer]]s (''n''-butane and isobutane) of [[butane]]. |
||
The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: |
The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: |
||
* If it connects at one of the two terminal |
* If it connects at one of the two terminal carbon [[atom]]s, it is '''normal butyl''' or '''''n''-butyl''': {{chem2|\sCH2\sCH2\sCH2\sCH3}} (preferred IUPAC name: '''butyl''') |
||
* If it connects at one of the non-terminal (internal) carbon atoms, it is '''secondary butyl''' or '''''sec''-butyl''': {{chem2| |
* If it connects at one of the non-terminal (internal) carbon atoms, it is '''secondary butyl''' or '''''sec''-butyl''': {{chem2|\sCH(CH3)\sCH2\sCH3}} (preferred IUPAC name: butan-2-yl) |
||
The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: |
The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: |
||
* If it connects at one of the three terminal carbons, it is '''isobutyl''': {{chem2| |
* If it connects at one of the three terminal carbons, it is '''isobutyl''': {{chem2|\sCH2\sCH(CH3)2}} (preferred IUPAC name: 2-methylpropyl) |
||
* If it connects at the central carbon, it is '''tertiary butyl''', '''''tert''-butyl''' or '''''t''-butyl''': ( |
* If it connects at the central carbon, it is '''tertiary butyl''', '''''tert''-butyl''' or '''''t''-butyl''': {{chem2|\sC(CH3)3}} (preferred IUPAC name: ''tert''-butyl) |
||
== Nomenclature == |
== Nomenclature == |
||
According to [[IUPAC nomenclature]], "isobutyl", "''sec''-butyl", and "''tert''-butyl" used to be allowed retained names. The latest guidance changed that: only ''tert''-butyl is kept as preferred prefix, all other butyl-names are removed. |
According to [[IUPAC nomenclature]], "isobutyl", "''sec''-butyl", and "''tert''-butyl" used to be allowed retained names. The latest guidance changed that: only ''tert''-butyl is kept as preferred prefix, all other butyl-names are removed. In the convention of [[skeletal formula]]s, every line ending and line intersection specifies a carbon atom (unless otherwise indicated) saturated with single-linked [[hydrogen]] atoms (unless otherwise indicated). The "R" symbol indicates any [[Radical (chemistry)|radical]] or other non-specific functional group. |
||
{| class="wikitable" |
{| class="wikitable" |
||
![[Skeletal formula]] |
![[Skeletal formula]] of butyl<br>(here connected to an R group) |
||
![[Common name]] |
![[Trivial name|Common name]] |
||
!Preferred<br />IUPAC name |
!Preferred<br />[[IUPAC]] name |
||
| ⚫ | |||
! |
! Fully [[systematic name]] |
||
| ⚫ | |||
! [[Skeletal formula#Alkyl groups|Symbol]] |
|||
|- |
|- |
||
|[[File:N-Butyl-Skeletal-SVG.svg|100px]] |
|[[File:N-Butyl-Skeletal-SVG.svg|100px]] |
||
| Line 30: | Line 31: | ||
|butyl |
|butyl |
||
|butan-1-yl |
|butan-1-yl |
||
|Bu, ''n''-Bu, ''n''Bu, <sup>''n''</sup>Bu |
|||
|- |
|- |
||
|[[File:Sec-Butyl-Skeletal-SVG.svg|80px]] |
|[[File:Sec-Butyl-Skeletal-SVG.svg|80px]] |
||
| Line 36: | Line 38: | ||
|1-methylpropyl |
|1-methylpropyl |
||
|butan-2-yl |
|butan-2-yl |
||
|''s''-Bu, ''s''Bu, <sup>''s''</sup>Bu |
|||
|- |
|- |
||
|[[File:Isobutyl-Skeletal-SVG.svg|80px]] |
|[[File:Isobutyl-Skeletal-SVG.svg|80px]] |
||
|isobutyl |
|isobutyl, ''iso''-butyl |
||
|2-methylpropyl |
|2-methylpropyl |
||
|2-methylpropyl |
|2-methylpropyl |
||
|2-methylpropan-1-yl |
|2-methylpropan-1-yl |
||
|''i''-Bu, ''i''Bu, <sup>''i''</sup>Bu |
|||
|- |
|- |
||
|[[File:Tert-Butyl-Skeletal-SVG.svg|60px]] |
|[[File:Tert-Butyl-Skeletal-SVG.svg|60px]] |
||
| Line 48: | Line 52: | ||
|1,1-dimethylethyl |
|1,1-dimethylethyl |
||
|2-methylpropan-2-yl |
|2-methylpropan-2-yl |
||
|''t''-Bu, ''t''Bu, <sup>''t''</sup>Bu |
|||
|} |
|} |
||
Butyl is the largest substituent for which [[trivial name]]s are commonly used for all isomers. |
Butyl is the largest substituent for which [[trivial name]]s are commonly used for all isomers. |
||
The butyl group's carbon that is connected to the rest (R) of the molecule is called the R<sup>I</sup> or R-prime carbon {{Citation needed|date=January 2011}}. The prefixes ''sec'' (from "secondary") and ''tert'' (from "tertiary") refer to the number of additional [[side chain]]s (or carbons) connected to the first butyl carbon. The prefix "iso" |
The butyl group's carbon that is connected to the rest (R) of the molecule is called the R<sup>I</sup> or R-prime carbon {{Citation needed|date=January 2011}}. The prefixes ''sec'' (from "secondary") and ''tert'' (from "tertiary") refer to the number of additional [[side chain]]s (or carbons) connected to the first butyl carbon. The prefix "iso" or "''iso''" means "isolated" while the prefix <nowiki>'</nowiki>''n-''<nowiki>'</nowiki> stands for "normal". |
||
Butan-2-yl (''sec''-butyl) group is [[Chirality (chemistry)|chiral]]. The carbon atom at position 2 is a [[stereocenter]]. It has four different groups attached: {{chem2|\sH}}, {{chem2|\sCH3}}, {{chem2|\sCH2\sCH3}}, and {{chem2|\sR}} (the R group is not equal to those three groups). The names of the two chiral groups are: (2''S'')-butan-2-yl and (2''R'')-butan-2-yl. |
|||
== Example == |
== Example == |
||
The four isomers of "[[butyl acetate]]" demonstrate these four isomeric configurations. |
The four isomers (ignoring [[stereoisomer]]s) of "[[butyl acetate]]" demonstrate these four isomeric configurations. Here, the acetate radical appears in each of the positions where the "R" symbol is used in the chart above: |
||
{| |
{| |
||
|[[File: |
|[[File:Butylacetat.svg|200px|butyl acetate]] |
||
|[[File: |
|[[File:Sec-butyl acetate.svg|150px|sec-butyl acetate]] |
||
|[[File:Isobutyl acetate. |
|[[File:Isobutyl acetate.svg|150px|isobutyl acetate]] |
||
|[[File: |
|[[File:Tert-Butylacetat.svg|120px|tert-butyl acetate]] |
||
|- |
|- |
||
| |
|{{center|[[Butyl acetate|''n''-butyl acetate]]}} |
||
| |
|{{center|[[Sec-Butyl acetate|''sec''-butyl acetate]]}} |
||
| |
|{{center|[[isobutyl acetate]]}} |
||
| |
|{{center|[[Tert-Butyl acetate|''tert''-butyl acetate]]}} |
||
|} |
|} |
||
''sec''-Butyl acetate is chiral, and has one stereocenter, and two [[enantiomer]]s. The names of enantiomers are: |
|||
* [(2''S'')-butan-2-yl] acetate, (+)-''sec''-Butyl acetate |
|||
* [(2''R'')-butan-2-yl] acetate, (-)-''sec''-Butyl acetate |
|||
Therefore, for butyl acetate, the total number of isomers is five, if stereoisomers are included. |
|||
== Etymology == |
== Etymology == |
||
Alkyl radicals are often considered as a series, a progression sequenced by the number of carbon atoms involved. In that progression, Butyl (containing 4 carbon atoms) is the fourth, and the last |
Alkyl radicals are often considered as a series, a progression sequenced by the number of carbon atoms involved. In that progression, Butyl (containing 4 carbon atoms) is the fourth, and the last with preferred IUPAC name derived from its history. The word "butyl" is derived from [[butyric acid]], a four-carbon [[carboxylic acid]] found in [[Rancidification|rancid]] [[butter]].<ref>{{Cite web|url = http://www.dictionary.com/cite.html?qh=butane&ia=etymon2|title = butane. Dictionary.com|last = Harper|first = Douglas|access-date = 9 Mar 2016}}{{Dead link|date=October 2019 |bot=InternetArchiveBot |fix-attempted=yes}}</ref> The name "butyric acid" comes from [[Latin]] ''butyrum'', '''butter'''. Subsequent preferred IUPAC names for alkyl radicals in the series are simply named from the [[Greek language|Greek]] number that indicates the number of carbon atoms in the group: [[pentyl]], [[Hexane|hexyl]], [[Heptane|heptyl]], etc. |
||
== ''tert''-Butyl effect == |
== ''tert''-Butyl effect == |
||
The ''tert''-butyl [[substituent]] is very bulky and is used in chemistry for [[kinetic reaction control|kinetic stabilization]], as are other bulky groups such as the related [[trimethylsilyl]] group. The effect of the ''tert''-butyl group on the progress of a chemical reaction is called the |
The ''tert''-butyl [[substituent]] is very bulky and is used in chemistry for [[kinetic reaction control|kinetic stabilization]], as are other bulky groups such as the related [[trimethylsilyl]] group. The effect of the ''tert''-butyl group on the progress of a chemical reaction is called the [[Thorpe–Ingold effect]] illustrated in the [[Diels-Alder reaction]] below. Compared to a hydrogen substituent, the ''tert''-butyl substituent accelerates the [[reaction rate]] by a factor of 240.<ref>''Factors affecting ease of ring formation. The effect of anchoring substitution on the rate of an intramolecular diels-alder reaction with furan-diene'' Serge Cauwberghs and Pierre J. De Clercq B. Tinant and J. P. Declercq [[Tetrahedron Letters]] Volume 29, Issue 20 , '''1988''', Pages 2493-2496 {{doi|10.1016/S0040-4039(00)87916-2}}</ref> |
||
[[File:Tertbutyleffect.svg|center|400px|''tert''-Butyl effect]] |
[[File:Tertbutyleffect.svg|center|400px|''tert''-Butyl effect]] |
||
The ''tert''-butyl effect is an example of [[Steric effects#Steric hindrance|steric hindrance]]. |
The ''tert''-butyl effect is an example of [[Steric effects#Steric hindrance|steric hindrance]]. |
||
== ''tert''-Butyl as protecting group == |
|||
A ''tert''-butyl (''t''Bu) ether is an acid-labile protecting group for alcohols.<ref name=":0">{{cite book|last1=Wuts|first1=P. G. M.|last2=Greene, T.W.|year=2006|title=Greene's Protective Groups in Organic Synthesis|doi=10.1002/0470053488|isbn= 9780470053485|publisher=J. Wiley|location=NY}}</ref> |
|||
===Protection=== |
|||
A traditional way to introduce the ''t''Bu group to a hydroxyl group is by treating the compound with [[isobutylene]] in the presence of a [[Brønsted acid]] or [[Lewis acid]].<ref>{{cite journal |last1=Micheli |first1=Robert A. |last2=Hojos |first2=Zoltan G. |last3=Cohen |first3=Noal |last4=Parrish |first4=David R. |last5=Portland |first5=Louis A. |last6=Sciamanna |first6=Werner |last7=Scott |first7=Melinda A. |last8=Wehrli |first8=Pius A. |title=Total syntheses of optically active 19-nor steroids. (+)-Estr-4-ene-3,17-dione and (+)-13.beta.-ethylgon-4-ene-3,17-dione |journal=The Journal of Organic Chemistry |date=March 1975 |volume=40 |issue=6 |pages=675–681 |doi=10.1021/jo00894a003 |url=https://doi.org/10.1021/jo00894a003 |language=en |issn=0022-3263}}</ref><ref>{{cite journal |title=Proceedings of the Chemical Society. July 1961 |journal=Proceedings of the Chemical Society |date=1 January 1961 |issue=July |pages=249 |doi=10.1039/PS9610000229 |url=https://doi.org/10.1039/PS9610000229 |language=en |issn=0369-8718}}</ref> |
|||
===Deprotection=== |
|||
Various acids can be used to cleave the ''t''Bu group, including both Brønsted acids such as [[trifluoroacetic acid]] and Lewis acids such as [[titanium tetrachloride]].<ref name=":0" /> |
|||
== References == |
== References == |
||
Latest revision as of 22:07, 1 March 2024
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula −C4H9, derived from either of the two isomers (n-butane and isobutane) of butane.
The isomer n-butane can connect in two ways, giving rise to two "-butyl" groups:
- If it connects at one of the two terminal carbon atoms, it is normal butyl or n-butyl: −CH2−CH2−CH2−CH3 (preferred IUPAC name: butyl)
- If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or sec-butyl: −CH(CH3)−CH2−CH3 (preferred IUPAC name: butan-2-yl)
The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups:
- If it connects at one of the three terminal carbons, it is isobutyl: −CH2−CH(CH3)2 (preferred IUPAC name: 2-methylpropyl)
- If it connects at the central carbon, it is tertiary butyl, tert-butyl or t-butyl: −C(CH3)3 (preferred IUPAC name: tert-butyl)
Nomenclature
According to IUPAC nomenclature, "isobutyl", "sec-butyl", and "tert-butyl" used to be allowed retained names. The latest guidance changed that: only tert-butyl is kept as preferred prefix, all other butyl-names are removed. In the convention of skeletal formulas, every line ending and line intersection specifies a carbon atom (unless otherwise indicated) saturated with single-linked hydrogen atoms (unless otherwise indicated). The "R" symbol indicates any radical or other non-specific functional group.
| Skeletal formula of butyl (here connected to an R group) |
Common name | Preferred IUPAC name |
Alternate notation | Fully systematic name | Symbol |
|---|---|---|---|---|---|
| n-butyl | butyl | butyl | butan-1-yl | Bu, n-Bu, nBu, nBu | |

|
sec-butyl | butan-2-yl | 1-methylpropyl | butan-2-yl | s-Bu, sBu, sBu |
| isobutyl, iso-butyl | 2-methylpropyl | 2-methylpropyl | 2-methylpropan-1-yl | i-Bu, iBu, iBu | |

|
tert-butyl | tert-butyl | 1,1-dimethylethyl | 2-methylpropan-2-yl | t-Bu, tBu, tBu |
Butyl is the largest substituent for which trivial names are commonly used for all isomers.
The butyl group's carbon that is connected to the rest (R) of the molecule is called the RI or R-prime carbon [citation needed]. The prefixes sec (from "secondary") and tert (from "tertiary") refer to the number of additional side chains (or carbons) connected to the first butyl carbon. The prefix "iso" or "iso" means "isolated" while the prefix 'n-' stands for "normal".
Butan-2-yl (sec-butyl) group is chiral. The carbon atom at position 2 is a stereocenter. It has four different groups attached: −H, −CH3, −CH2−CH3, and −R (the R group is not equal to those three groups). The names of the two chiral groups are: (2S)-butan-2-yl and (2R)-butan-2-yl.
Example
The four isomers (ignoring stereoisomers) of "butyl acetate" demonstrate these four isomeric configurations. Here, the acetate radical appears in each of the positions where the "R" symbol is used in the chart above:
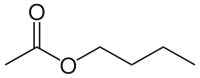
|

|
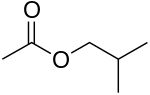
|
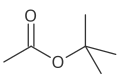
|
sec-Butyl acetate is chiral, and has one stereocenter, and two enantiomers. The names of enantiomers are:
- [(2S)-butan-2-yl] acetate, (+)-sec-Butyl acetate
- [(2R)-butan-2-yl] acetate, (-)-sec-Butyl acetate
Therefore, for butyl acetate, the total number of isomers is five, if stereoisomers are included.
Etymology
Alkyl radicals are often considered as a series, a progression sequenced by the number of carbon atoms involved. In that progression, Butyl (containing 4 carbon atoms) is the fourth, and the last with preferred IUPAC name derived from its history. The word "butyl" is derived from butyric acid, a four-carbon carboxylic acid found in rancid butter.[1] The name "butyric acid" comes from Latin butyrum, butter. Subsequent preferred IUPAC names for alkyl radicals in the series are simply named from the Greek number that indicates the number of carbon atoms in the group: pentyl, hexyl, heptyl, etc.
tert-Butyl effect
The tert-butyl substituent is very bulky and is used in chemistry for kinetic stabilization, as are other bulky groups such as the related trimethylsilyl group. The effect of the tert-butyl group on the progress of a chemical reaction is called the Thorpe–Ingold effect illustrated in the Diels-Alder reaction below. Compared to a hydrogen substituent, the tert-butyl substituent accelerates the reaction rate by a factor of 240.[2]
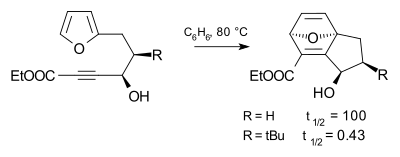
The tert-butyl effect is an example of steric hindrance.
tert-Butyl as protecting group
A tert-butyl (tBu) ether is an acid-labile protecting group for alcohols.[3]
Protection
A traditional way to introduce the tBu group to a hydroxyl group is by treating the compound with isobutylene in the presence of a Brønsted acid or Lewis acid.[4][5]
Deprotection
Various acids can be used to cleave the tBu group, including both Brønsted acids such as trifluoroacetic acid and Lewis acids such as titanium tetrachloride.[3]
References
- ^ Harper, Douglas. "butane. Dictionary.com". Retrieved 9 Mar 2016.[permanent dead link]
- ^ Factors affecting ease of ring formation. The effect of anchoring substitution on the rate of an intramolecular diels-alder reaction with furan-diene Serge Cauwberghs and Pierre J. De Clercq B. Tinant and J. P. Declercq Tetrahedron Letters Volume 29, Issue 20 , 1988, Pages 2493-2496 doi:10.1016/S0040-4039(00)87916-2
- ^ a b Wuts, P. G. M.; Greene, T.W. (2006). Greene's Protective Groups in Organic Synthesis. NY: J. Wiley. doi:10.1002/0470053488. ISBN 9780470053485.
- ^ Micheli, Robert A.; Hojos, Zoltan G.; Cohen, Noal; Parrish, David R.; Portland, Louis A.; Sciamanna, Werner; Scott, Melinda A.; Wehrli, Pius A. (March 1975). "Total syntheses of optically active 19-nor steroids. (+)-Estr-4-ene-3,17-dione and (+)-13.beta.-ethylgon-4-ene-3,17-dione". The Journal of Organic Chemistry. 40 (6): 675–681. doi:10.1021/jo00894a003. ISSN 0022-3263.
- ^ "Proceedings of the Chemical Society. July 1961". Proceedings of the Chemical Society (July): 249. 1 January 1961. doi:10.1039/PS9610000229. ISSN 0369-8718.













