Potency and safety analysis of hemp-derived delta-9 products: The hemp vs. cannabis demarcation problem
Contents
| Wound | |
|---|---|
 | |
| Hand abrasion resulting from a bicycle accident | |
| Specialty | |
A wound is any disruption of or damage to living tissue, such as skin, mucous membranes, or organs.[1][2] Wounds can either be the sudden result of direct trauma (mechanical, thermal, chemical), or can develop slowly over time due to underlying disease processes such as diabetes mellitus, venous/arterial insufficiency, or immunologic disease.[3] Wounds can vary greatly in their appearance depending on wound location, injury mechanism, depth of injury, timing of onset (acute vs chronic), and wound sterility, among other factors.[1][2] Treatment strategies for wounds will vary based on the classification of the wound, therefore it is essential that wounds be thoroughly evaluated by a healthcare professional for proper management. In normal physiology, all wounds will undergo a series of steps collectively known as the wound healing process, which include hemostasis, inflammation, proliferation, and tissue remodeling. Age, tissue oxygenation, stress, underlying medical conditions, and certain medications are just a few of the many factors known to affect the rate of wound healing.[4]
Classification
Wounds can be broadly classified as either acute or chronic based on time from initial injury and progression through normal stages of wound healing. Both wound types can further be categorized by cause of injury, wound severity/depth, and sterility of the wound bed. Several classification systems have been developed to describe wounds and guide their management. Some notable classification systems include the CDC's Surgical Wound Classification, the International Red Cross Wound Classification, the Tscherne classification, the Gustilo-Anderson classification of open fractures, and the AO soft tissue grading system.[2][5]
Acute wounds
An acute wound is any wound which results from direct trauma and progresses through the four stages of wound healing along an expected timeline. The first stage, hemostasis, lasts from minutes to hours after initial injury. This stage is followed by the inflammatory phase which typically lasts 1 to 3 days. Proliferation is the third stage of wound healing and lasts from a few days up to a month. The fourth and final phase of wound healing, remodeling/scar formation, typically lasts 12 months but can continue as long as 2 years after the initial injury.[6][7] Acute wounds can further be classified as either open or closed. An open wound is any injury whereby the integrity of the skin has been disrupted and the underlying tissue is exposed. A closed wound, on the other hand, is any injury in which underlying tissue has been damaged but the overlying skin is still intact.[8]
Open wounds
- Incisions or incised wounds – caused by a clean, sharp-edged object such as a knife, razor, or glass splinter.[citation needed]
- Lacerations – irregular tear-like wounds caused by some blunt trauma. Lacerations and incisions may appear linear (regular) or stellate (irregular). The term laceration is commonly misused in reference to incisions.[9]
- Abrasions (grazes) – superficial wounds in which the topmost layer of the skin (the epidermis) is scraped off. Abrasions are often caused by a sliding fall onto a rough surface such as asphalt, tree bark or concrete.[citation needed]
- Avulsions – injuries in which a body structure is forcibly detached from its normal point of insertion; a type of amputation where the extremity is pulled off rather than cut off. When used in reference to skin avulsions, the term 'degloving' is also sometimes used as a synonym.[citation needed]
- Puncture wounds – caused by an object puncturing the skin, such as a splinter, nail, knife or sharp tooth.[10]
- Penetration wounds – caused by an object such as a knife entering and coming out from the skin.[citation needed]
- Gunshot wounds – caused by a bullet or similar projectile driving into or through the body. There may be two wounds, one at the site of entry and one at the site of exit, generally referred to as a "through-and-through."[citation needed]
- Critical wounds – Including large burns that have been split. These wounds can cause serious hydroelectrolytic and metabolic alterations including fluid loss, electrolyte imbalances, and increased catabolism.[11][12][13]
Closed wounds
- Hematomas (or blood tumor) – caused by damage to a blood vessel that in turn causes blood to collect under the skin.[citation needed]
- Hematomas that originate from internal blood vessel pathology are petechiae, purpura, and ecchymosis. The different classifications are based on size.[citation needed]
- Hematomas that originate from an external source of trauma are contusions, also commonly called bruises.
- Crush injury – caused by a great or extreme amount of force applied over a long period of time.[citation needed]
-
An open wound (an avulsion)
-
A laceration to the leg
-
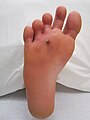
An infected puncture wound to the bottom of the forefoot
-
A puncture wound from playing darts
-
An incision: a small cut in a finger
-
Fresh incisional wound on the fingertip of the left ring finger
-
Abrasion on knee
-
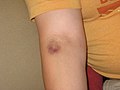
Bruise on arm
Fractures
Fractures can be classified as either open or closed, depending on whether the integrity of the overlying skin has been disrupted or preserved, respectively. Several classification systems have been developed to further characterize soft tissue injuries in the setting of an underlying fracture:[14]
- Tscherne classification – Used to describe external appearance of wounds in both open and closed fractures.
- Gustilo-Anderson classification – Classifies open fractures based on wound size, extent of soft tissue loss, and degree of contamination.[15]
- Hannover Fracture scale – Used in open fractures as an extremity salvage assessment.
- AO Classification – adapted from the Tscherne classification, provides separate grading system for skin, muscles/tendons, and neurovascular structures.[16]
Chronic wounds
Any wound which is arrested or delayed during any of the normal stages of wound healing is considered to be a chronic wound. Most commonly, these are wounds which develop due to an underlying disease process such as diabetes mellitus or arterial/venous insufficiency. However, it is important to note that any acute wound has the potential to become a chronic wound if any of the normal stages of wound healing are interrupted. Chronic wounds are most commonly a result of disruption of the inflammatory phase of wound healing, however errors in any phase can result in a chronic wound.[1] The exact duration of time which distinguishes a chronic wound from an acute wound is not clearly defined, although many clinicians agree that wounds which have not progressed for over three months are considered chronic wounds.[1][17]
Common causes of chronic wounds
- Diabetes mellitus[18] – Wound healing impairment in the setting of diabetes is multifactorial. Hyperglycemia, neuropathy, microvascular complications, impaired immune and inflammatory responses, and psychological factors have all been implicated in the formation and propagation of diabetic wounds. Feet are the most common location of diabetic wounds, although any type of wound can be negatively impacted by diabetes. It has been estimated that up to 25% of patients with diabetes mellitus will be affected by non-healing wounds in their lifetime.
- Venous/Arterial insufficiency[17][19][20] – Impaired blood outflow (venous) or inflow (arterial) can both impair wound healing, thereby causing chronic wounds. Much like diabetes, venous/arterial insufficiency most commonly result in chronic wounds of the lower extremities. In chronic venous insufficiency, blood pooling impedes oxygen exchange and creates a chronic pro-inflammatory environment which both promote formation of venous ulcers. Peripheral artery disease, on the other hand, causes wounds due to poor blood inflow and typically affects the most distal extremities (fingers, toes).
- Immunologic disease[21] – The immune system plays a critical role in the inflammatory process; therefore, any disease of the immune system has the potential to impair the inflammatory phase of wound healing, thereby leading to a chronic wound. Patients suffering from diseases such as rheumatoid arthritis and lupus have been found to have larger wounds and prolonged time to heal when compared to the general population.
- Pressure ulcer[22] – Also known as decubitus ulcers or bedsores, this type of wound is a result of chronic pressure to the skin over a prolonged period. While most individuals have intact sensation and motor function which allow for frequent positional change to prevent the formation of such ulcers, older individuals are particularly susceptible to this type of chronic injury due to impaired neurosensory responses. Pressure ulcers can occur in as little as two hours of immobility in a bedridden patient or person who is otherwise unconscious/sedated (surgery, syncope, etc.). In the United States, pressure ulcers are graded using the National Pressure Injury Advisory Panel (NPIAP) system. In this system, ulcers are graded on wound depth with stage 1 being the least severe (erythema, intact skin) and stage 4 being full thickness damage through subcutaneous tissue down to muscle, tendon, or bone. Any ulcer that cannot be assessed due to overlying eschar is considered unstageable.
Wound sterility
Wound sterility, or degree of contamination of a wound, is a critical consideration when evaluating a wound. In the United States, the CDC's Surgical Wound Classification System is most commonly used for classification of a wound's sterility, specifically within a surgical setting. According to this classification system, four different classes of wound exist, each with their own postoperative risk of surgical site infection:[2][23]
- Class 1 – clean wound: a wound that is not infected and without signs of inflammation. This type of wound is typically closed. By definition, this type of wound excludes any wounds of the respiratory, genital, alimentary, or urinary tract.
- Class 2 – clean-contaminated wound: a wound with a low level of contamination. May involve entry into the respiratory, genital, alimentary, or urinary tract.
- Class 3 – contaminated wound: an open, accidental wound resulting from trauma outside of a sterile setting is automatically considered a contaminated wound. Additionally, any surgical wound where there is a major break in sterile technique or obvious contamination from the gastrointestinal tract is considered a contaminated wound.
- Class 4 – dirty/infected: a wound with evidence of an existing clinical infection. Class 4 wounds are usually found in old traumatic wounds which were not adequately treated and will show evidence of devitalized tissue or gross purulence.
Presentation
Workup

Physical examination
Wound presentation will vary greatly based on a number of factors, each of which is important to consider in order to establish a proper diagnosis and treatment plan. In addition to collecting a thorough history, the following factors should be considered when evaluating any wound:[1][24]
- Size of wound: Should be accurately measured at time of initial presentation and regularly remeasured until wound resolution.
- Wound location: Very useful consideration in many chronic wounds, such as diabetic foot ulcers, pressure ulcers, and venous ulcers. Acute wounds will be located in areas consistent with the mechanism of injury (e.g. diagonal chest wall bruising from seatbelt following car accident).
- Wound bed: A healthy wound bed will appear pink due to healthy granulation tissue. Presence of a dark red wound bed which bleeds easily on contact or excess granulation tissue (i.e. hypergranulation tissue) may indicate the presence of an infection or non-healing wound.
- Wound depth: The depth of a wound is often not apparent on visual inspection alone. Proper evaluation of wound depth includes use of a probe to measure wound depth and evaluate for undermining of wound edges or sinus/fistula formation.
- Necrotic tissue, slough, eschar: Wounds may be covered with a layer of dead tissue which may appear cream/yellow in color (slough) or as a black, hardened tissue (eschar). Removing this tissue is critical for properly evaluating both the depth of a wound and quality of the wound bed, and promotes wound healing.
- Wound edges: May provide clues to cause of specific wounds, such as gently sloping edges of venous ulcers or rolled edges of certain tumors.
- Surrounding skin: Appearance of the surrounding skin can provide clues to underlying disease processes, such as redness/erythema due to cellulitis, maceration due to uncontrolled wound exudate, or eczematous changes due to a chronic irritation (e.g. allergic reaction to wound dressing).
- Infection: Classic signs of infection are redness, warmth, swelling, odor, and pain out of proportion to wound appearance.
- Pain: Pain can be nociceptive, neuropathic, or inflammatory, each of which can provide clues to the cause of a wound.[25] Proper pain control is an important consideration in wound management, particularly in burn care where analgesia is often necessary prior to dressing changes.
A thorough wound evaluation, particularly evaluation of wound depth and removal of necrotic tissue, should be performed only by a licensed healthcare professional in order to avoid damage to nearby structures, infection, or worsening pain.[citation needed]
Diagnostics
Additional diagnostic tests may be needed during wound evaluation based on the cause, appearance, and age of a wound.[1][26]
- Wound culture: If there is concern for infection, a wound can be more carefully evaluated for presence of bacteria via surface swabs, deep tissue biopsy, or needle biopsy. Surface swabs are most commonly used due to low cost, ease of use, and minimal pain to patient. Although swab cultures have been shown to reliably identify the organisms causing an infection, swabs are only able to identify bacteria on the surface of a wound and can occasionally be contaminated by normal skin flora. Deep tissue biopsy is considered the gold standard for diagnosing wound infections due to being both more accurate and precise than swabs, however it is more invasive, more painful, and less cost effective than swabs and therefore is not the first choice for collecting wound cultures. Needle aspiration can only be implemented in wounds with underlying abscesses or fluid collections.
- Imaging: X-ray is useful to assess for an underlying fracture which may not be apparent on physical examination alone. Ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) can all be used to assess for identifying fluid collections, necrotic tissue, or inflammation. Ultrasound is portable, low cost, quickly implemented, and does not expose patients to radiation, but is limited in diagnostic capabilities. CT is another quickly implemented option which generally provides more diagnostic information compared to ultrasound, however it is less cost-effective and exposes patients to radiation. MRI offers the greatest image resolution and can provide diagnostic information on presence of soft tissue infection or bone infection. Like ultrasound, MRI does not expose patients to radiation, however it is the slowest and most difficult to implement of the all of these imaging methods.
- Laboratory studies: Serum prealbumin levels may be useful in evaluating nutrition status in patients with chronic wounds or at risk for developing chronic wounds. Elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) can confirm presence of an infection but alone are not diagnostic. Routine bloodwork such as a basic metabolic panel (BMP) or complete blood count (CBC) are not typically required but may be useful in select circumstances.
- Ankle-brachial index/toe-brachial index (ABI/TBI): These tests can be used to assess blood supply to the lower extremities and their results may affect management of lower extremity wounds such as venous/arterial ulcers, diabetic foot ulcers, or pressure ulcers.
Management
The goal of wound care is to promote an environment that allows a wound to heal as quickly as possible, with emphasis on restoring both form and function of the wounded area. Although optimal treatment strategies vary greatly depending on the specific cause, size, and age of a particular wound, there are universal principles of wound management that apply to all wounds.[1] After a thorough evaluation is performed, all wounds should be properly irrigated and debrided.[27] Proper cleansing of a wound is critical to prevent infection and promote re-epithelialization. Further efforts should be made to eliminate/limit any contributing factors to the wound (e.g. diabetes, pressure, etc.) and optimize the wound's healing ability (i.e. optimize nutritional status).[1] The end goal of wound management is closure of the wound which can be achieved by primary closure, delayed primary closure, or healing by secondary intention, each of which is discussed below. Pain control is a mainstay of wound management, as wound evaluation, wound cleansing, and dressing changes can be a painful process.[27]
Irrigation
Proper cleansing of a wound is critical in preventing infection and promoting healing of any wound. Irrigation is defined as constant flow of a solution over the surface of a wound. The goal of irrigation is not only to remove debris and potential contaminants from a wound, but also to assist in visual inspection of a wound and hydrate the wound.[27] Irrigation is typically achieved with either a bulb or syringe and needle/catheter. The preferred solution for irrigation is normal saline which is readily accessible in the emergency department, although recent studies have shown no difference in emergency department infection rates when comparing normal saline to potable tap water.[28] Irrigation can also be achieved with a diluted 1% povidone iodine solution, but studies have again shown no difference in infection rates when compared to normal saline.[29] Irrigation with antiseptic solutions, such as non-diluted povidone iodine, chlorhexidine, and hydrogen peroxide is not preferred since these solutions are toxic to tissue and inhibit wound healing. The exact volume of irrigation used will vary depending on the appearance of the wound, although some sources have reported 50–100 mL of irrigation per 1 cm of wound length as a guideline.[27]
Debridement
Debridement is defined as removal of devitalized or dead tissue, particularly necrotic tissue, eschar, or slough. Debridement is a critical aspect of wound care because devitalized tissue, particularly necrotic tissue, serves as nutrients for bacteria thereby promoting infection. Additionally, devitalized tissue creates a physical barrier over a wound which limits the effectiveness of any applied topical compounds and prevents re-epithelialization. Lastly, devitalized tissue, especially eschar, prevents accurate assessment of underlying tissue, making appropriate assessment of a wound impossible without adequate debridement. Debridement can be achieved in several ways:[30]
- Autolytic debridement: The most conservative type of debridement whereby the body's own natural defenses break down necrotic tissue via phagocytes and proteolytic enzymes. This method requires a moist environment and intact immune system.
- Mechanical debridement: Achieved through use of mechanical force to remove devitalized tissue (e.g. wet-to-dry dressing, pressurized wound irrigation, pulse-lavage); however, this process will remove both healthy and non-healthy tissue and is therefore considered a non-selective debridement method.
- Enzymatic debridement: A process of debridement in which enzymes such as proteinases or collagenases are applied topically to digest devitalized tissue. Depending on the agent, this process can be either selective or non-selective. Examples include trypsin, streptokinase-streptodornase combination, subtilisin, papain, and collagenase.[31]
- Surgical debridement: Also known as sharp debridement, this is a process in which devitalized tissue is removed through use of surgical instruments such as scalpels, curettes, or surgical scissors. Surgical debridement can be done in a hospital bed, in an outpatient clinic, or in an operating room depending on the particular wound, risk of bleeding, and anesthesia requirements.
- Biological debridement: Also known as larval therapy, biological debridement is done through controlled application of sterile larvae (Lucilia sericata) to the wound bed. These larvae release proteolytic enzymes which dissolve necrotic tissue before then ingesting the now debrided tissue. Biologic debridement has the added benefit of being bactericidal since larvae will ingest bacteria as well as devitalized tissue. Despite the safety and effectiveness of this method, its applications are often limited due to patient's negative feelings towards larvae which are commonly associated with poor hygiene and perishable food.[32]
Closure

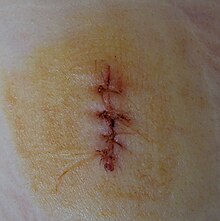
The end goal of wound care is to re-establish the integrity of the skin, a structure which serves as a barrier to the external environment.[33] The preferred method of closure is to reattach/reapproximate the wound edges together, a process known as primary closure/healing by primary intention. Wounds that have not been closed within several hours of the initial injury or wounds that are concerning for infection will often be left open and treated with dressings for several days before being closed 3–5 days later, a process known as delayed primary closure. The exact duration of time from initial injury in which delayed primary closure is preferred over primary closure is not clearly defined.[34] Wounds that cannot be closed primarily due to substantial tissue loss can be healed by secondary intention, a process in which the wound is allowed to fill-in over time through natural physiologic processes. When healing by secondary intention, granulation tissue grows in from the wound edges slowly over time to restore integrity of the skin. Healing by secondary intention can take up to months, requires daily wound care, and leaves an unfavorable scar, thus primary closure is always preferred when possible.[27][35] As an alternative, wounds that cannot be closed primarily can be addressed with skin grafting or flap reconstruction, typically done by a plastic surgeon.[33] There are several methods that can be implemented to achieve primary closure of a wound, including suture, staples, skin adhesive, and surgical strips. Suture is the most frequently used for closure.[27] There are many types of suture, but broadly they can be categorized as absorbable vs non-absorbable and synthetic vs natural. Absorbable sutures have the added benefit of not requiring removal and are often preferred in children for this reason.[36] Staples are less time-consuming and more cost effective than suture but have a risk of worse scarring if left in place for too long.[27] Adhesive glue and sutures have comparable cosmetic outcomes for minor lacerations <5 cm in adults and children.[37] The use of adhesive glue involves considerably less time for the doctor and less pain for the person. The wound opens at a slightly higher rate but there is less redness.[38] The risk for infections (1.1%) is the same for both. Adhesive glue should not be used in areas of high tension or repetitive movements, such as joints or the posterior trunk.[37]
Dressings
After a wound is irrigated, debrided, and, if possible, closed, it should be dressed appropriately. The goals of a wound dressing are to act as a barrier to the outside environment, facilitate wound healing, promote hemostasis, and act as a form of mechanical debridement during dressing changes.[39] The ideal wound dressing maintains a moist environment to optimize wound healing but is also capable of absorbing excess fluid as to avoid skin maceration or bacterial growth.[33] Several wound dressing options are available, each tailored to different kinds of wounds:[40]
- Gauze: Composed of woven or non-woven cotton, rayon, and polyester, gauze is highly absorbent, but removal can be uncomfortable.
- Films: Films are made of translucent polyurethane which is adherent to skin and semi-occlusive, allowing them to retain within the dressing but also allow for exchange of gases such as oxygen and carbon dioxide. The translucent nature of this dressing makes monitoring wounds simple.
- Hydrocolloids: Consist of an outer, water-impermeable layer and an inner layer made of colloid. When the inner colloid layer comes in contact with liquid, it becomes a gel allowing the dressing to maintain a moist environment while simultaneously absorbing exudate. Hydrocolloids cause minimal pain on removal but are at increased risk of skin maceration and bacterial growth.
- Hydrogels: An insoluble, hydrophilic material with soothing properties which is useful in treating burn wounds, dry chronic wounds, and pressure ulcers. Like hydrocolloids, hydrogels are capable of retaining excess moisture leading to skin maceration and bacterial growth.
- Foams: A flexible material with a hydrophobic outer layer that shields liquid from the outside environment, while having a highly absorptive inner layer which is ideal for highly exuding wounds. Foams should not be used in dryer wounds that require exudate to stay moist.
- Alginates: Derived from seaweed, alginates can absorb up to 15–20 times their weight in liquid and are ideal for highly exudative wounds. Like hydrocolloids, alginates form a gel when they come in contact with fluid, making removal relatively painless.
- Hydrofibers: A derivative of hydrocolloid dressings, hydrofibers are able to absorb up to 25 times their weight in fluid, making them the most absorbent dressing. They are much like alginate dressings in their absorptive capacity and tendency to form a gel upon contact with liquid.
- Medicated dressings: Many dressings come impregnated with medication, typically antimicrobial agents or debriding chemicals. Silver, iodine, growth hormones, enzymes, and antibacterial agents are most common.
- Negative-pressure wound therapy (NPWT):[41][42] A unique type of dressing which consists of a foam dressing surrounded with an airtight film and then connected to power-assisted vacuum suction, creating a negative pressure environment over the wound. This negative pressure environment is thought to promote formation of granulation tissue and decrease inflammatory fluid. NPWT has the added benefit of requiring less frequent dressing changes, a process that is often painful for patients. Since its implementation, NPWT has been implemented broadly for chronic non-healing wounds but can also be applied to acute wounds that cannot be closed primarily due to swelling or concern for infection. This type of dressing is typically applied in the operating room but can be done at bedside with appropriate analgesia.
Maintenance and surveillance
Ideally, wound dressings should be changed daily to promote a clean environment and allow for daily evaluation of wound progression. Highly exudative wounds and infected wounds should be monitored closely and may require more frequent dressing changes.[33] Negative pressure wound dressings can be changed less frequently, every 2–3 days.[42] Wound progression over time can be monitored with transparent sheet tracings or photographs, each of which produce reliable measurements of wound surface area.[33][43]
Alternative medicine
There is moderate evidence that honey is more effective than antiseptic followed by gauze for healing wounds infected after surgical operations. There is a lack of quality evidence relating to the use of honey on other types of wounds, such as minor acute wounds, mixed acute and chronic wounds, pressure ulcers, Fournier's gangrene, venous leg ulcers, diabetic foot ulcers and Leishmaniasis.[44]
Therapeutic touch has been implicated as a complementary therapy in wound healing; however, there is no high quality research supporting its use as an evidence based clinical intervention. [45] More than 400 species of plants are identified as potentially useful for wound healing.[46] Only three randomized controlled trials, however, have been done for the treatment of burns.[47]
History

From the Classical Period to the Medieval Period, the body and the soul were believed to be intimately connected, based on several theories put forth by the philosopher Plato. Wounds on the body were believed to correlate with wounds to the soul and vice versa; wounds were seen as an outward sign of an inward illness. Thus, a man who was wounded physically in a serious way was said to be hindered not only physically but spiritually as well. If the soul was wounded, that wound may also eventually become physically manifest, revealing the true state of the soul.[48] Wounds were also seen as writing on the "tablet" of the body. Wounds acquired in war, for example, told the story of a soldier in a form which all could see and understand, and the wounds of a martyr told the story of their faith.[48]
Research
In humans and mice it has been shown that estrogen might positively affect the speed and quality of wound healing.[49]
See also
- European Wound Management Association
- International Red Cross Wound Classification System
- Wound bed preparation
References
- ^ a b c d e f g h Nagle SM, Stevens KA, Wilbraham SC (2023). "Wound Assessment". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 29489199. Retrieved 12 January 2024.
- ^ a b c d Herman TF, Bordoni B (2023). "Wound Classification". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 32119343. Retrieved 12 January 2024.
- ^ Kujath P, Michelsen A (March 2008). "Wounds - from physiology to wound dressing". Deutsches Ärzteblatt International. 105 (13): 239–248. doi:10.3238/arztebl.2008.0239. PMC 2696775. PMID 19629204.
- ^ Guo S, Dipietro LA (March 2010). "Factors affecting wound healing". Journal of Dental Research. 89 (3): 219–229. doi:10.1177/0022034509359125. PMC 2903966. PMID 20139336.
- ^ van Gennip L, Haverkamp FJ, Muhrbeck M, Wladis A, Tan EC (September 2020). "Using the Red Cross wound classification to predict treatment needs in children with conflict-related limb injuries: a retrospective database study". World Journal of Emergency Surgery. 15 (1): 52. doi:10.1186/s13017-020-00333-0. PMC 7501687. PMID 32948211.
- ^ Wallace HA, Basehore BM, Zito PM (2023). "Wound Healing Phases". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 29262065. Retrieved 19 January 2024.
- ^ Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A (May 2021). "Immunology of Acute and Chronic Wound Healing". Biomolecules. 11 (5): 700. doi:10.3390/biom11050700. PMC 8150999. PMID 34066746.
- ^ Chhabra S, Chhabra N, Kaur A, Gupta N (December 2017). "Wound Healing Concepts in Clinical Practice of OMFS". Journal of Maxillofacial and Oral Surgery. 16 (4): 403–423. doi:10.1007/s12663-016-0880-z. PMC 5628060. PMID 29038623.
- ^ American Academy of Pediatrics (2011). First Aid for Families. Jones & Bartlett. p. 39. ISBN 978-0763755522.
- ^ "Cuts and puncture wounds". MedlinePlus Medical Encyclopedia. U.S. National Library of Medicine. Retrieved 8 November 2023.
- ^ Rae L, Fidler P, Gibran N (October 2016). "The Physiologic Basis of Burn Shock and the Need for Aggressive Fluid Resuscitation". Critical Care Clinics. 32 (4): 491–505. doi:10.1016/j.ccc.2016.06.001. PMID 27600122.
- ^ Mecott GA, González-Cantú I, Dorsey-Treviño EG, Matta-Yee-Chig D, Saucedo-Cárdenas O, Montes de Oca-Luna R, et al. (April 2020). "Efficacy and Safety of Pirfenidone in Patients with Second-Degree Burns: A Proof-of-Concept Randomized Controlled Trial". Advances in Skin & Wound Care. 33 (4): 1–7. doi:10.1097/01.ASW.0000655484.95155.f7. PMID 32195729. S2CID 213193146.
- ^ Nielson CB, Duethman NC, Howard JM, Moncure M, Wood JG (2017). "Burns: Pathophysiology of Systemic Complications and Current Management". Journal of Burn Care & Research. 38 (1): e469–e481. doi:10.1097/BCR.0000000000000355. PMC 5214064. PMID 27183443.
- ^ Ibrahim DA, Swenson A, Sassoon A, Fernando ND (February 2017). "Classifications In Brief: The Tscherne Classification of Soft Tissue Injury". Clinical Orthopaedics and Related Research. 475 (2): 560–564. doi:10.1007/s11999-016-4980-3. PMC 5213932. PMID 27417853.
- ^ Kim PH, Leopold SS (November 2012). "In brief: Gustilo-Anderson classification. [corrected]". Clinical Orthopaedics and Related Research. 470 (11): 3270–3274. doi:10.1007/s11999-012-2376-6. PMC 3462875. PMID 22569719.
- ^ Kellam J (2018). "Fracture classification". In Buckley RE, Moran CG, Apivatthakakul T (eds.). AO Principles of Fracture Management: Vol. 1: Principles, Vol. 2: Specific fractures. Stuttgart: Georg Thieme Verlag. doi:10.1055/b-0038-160815. ISBN 978-3-13-242309-1.
- ^ a b Star A (December 2018). "Differentiating Lower Extremity Wounds: Arterial, Venous, Neurotrophic". Seminars in Interventional Radiology. 35 (5): 399–405. doi:10.1055/s-0038-1676362. PMC 6363550. PMID 30728656.
- ^ Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I (October 2021). "Diabetic Wound-Healing Science". Medicina. 57 (10): 1072. doi:10.3390/medicina57101072. PMC 8539411. PMID 34684109.
- ^ Robles-Tenorio A, Lev-Tov H, Ocampo-Candiani J (2023). "Venous Leg Ulcer". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 33620871. Retrieved 19 January 2024.
- ^ Zemaitis MR, Boll JM, Dreyer MA (2023). "Peripheral Arterial Disease". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 28613496. Retrieved 19 January 2024.
- ^ Avishai E, Yeghiazaryan K, Golubnitschaja O (March 2017). "Impaired wound healing: facts and hypotheses for multi-professional considerations in predictive, preventive and personalised medicine". The EPMA Journal. 8 (1): 23–33. doi:10.1007/s13167-017-0081-y. PMC 5471802. PMID 28620441.
- ^ Zaidi SR, Sharma S (2023). "Pressure Ulcer". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 31971747. Retrieved 19 January 2024.
- ^ Onyekwelu I, Yakkanti R, Protzer L, Pinkston CM, Tucker C, Seligson D (June 2017). "Surgical Wound Classification and Surgical Site Infections in the Orthopaedic Patient". Journal of the American Academy of Orthopaedic Surgeons. Global Research & Reviews. 1 (3): e022. doi:10.5435/JAAOSGlobal-D-17-00022. PMC 6132296. PMID 30211353.
- ^ Grey, Joseph E; Enoch, Stuart; Harding, Keith G (4 February 2006). "Wound assessment". BMJ. 332 (7536): 285–288. doi:10.1136/bmj.332.7536.285. ISSN 0959-8138. PMC 1360405. PMID 16455730.
- ^ Yam, Mun; Loh, Yean; Tan, Chu; Khadijah Adam, Siti; Abdul Manan, Nizar; Basir, Rusliza (24 July 2018). "General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation". International Journal of Molecular Sciences. 19 (8): 2164. doi:10.3390/ijms19082164. ISSN 1422-0067. PMC 6121522. PMID 30042373.
- ^ Li, Shuxin; Renick, Paul; Senkowsky, Jon; Nair, Ashwin; Tang, Liping (1 June 2021). "Diagnostics for Wound Infections". Advances in Wound Care. 10 (6): 317–327. doi:10.1089/wound.2019.1103. ISSN 2162-1918. PMC 8082727. PMID 32496977.
- ^ a b c d e f g Nicks, Bret A.; Ayello, Elizabeth A.; Woo, Kevin; Nitzki-George, Diane; Sibbald, R. Gary (December 2010). "Acute wound management: revisiting the approach to assessment, irrigation, and closure considerations". International Journal of Emergency Medicine. 3 (4): 399–407. doi:10.1007/s12245-010-0217-5. ISSN 1865-1372. PMC 3047833. PMID 21373312.
- ^ Fernandez, R.; Griffiths, R. (23 January 2008). Fernandez, Ritin (ed.). "Water for wound cleansing". The Cochrane Database of Systematic Reviews (1): CD003861. doi:10.1002/14651858.CD003861.pub2. ISSN 1469-493X. PMID 18254034.
- ^ Khan, Muhammad N.; Naqvi, Abul H. (November 2006). "Antiseptics, iodine, povidone iodine and traumatic wound cleansing". Journal of Tissue Viability. 16 (4): 6–10. doi:10.1016/S0965-206X(06)64002-3. PMID 17153117.
- ^ Manna, Biagio; Nahirniak, Phillip; Morrison, Christopher A. (2024). "Wound Debridement". StatPearls. Treasure Island, Florida: StatPearls Publishing. PMID 29939659. Retrieved 26 January 2024.
- ^ Nowak, Marcela; Mehrholz, Dorota; Barańska-Rybak, Wioletta; Nowicki, Roman (2022). "Wound debridement products and techniques: clinical examples and literature review". Advances in Dermatology and Allergology. 39 (3): 479–490. doi:10.5114/ada.2022.117572. ISSN 1642-395X. PMC 9326937. PMID 35950126.
- ^ Bazaliński, Dariusz; Przybek-Mita, Joanna; Pytlak, Kamila; Kardyś, Daria; Bazaliński, Adrian; Kucharzewski, Marek; Więch, Paweł (30 October 2023). "Larval Wound Therapy: Possibilities and Potential Limitations—A Literature Review". Journal of Clinical Medicine. 12 (21): 6862. doi:10.3390/jcm12216862. ISSN 2077-0383. PMC 10647679. PMID 37959326.
- ^ a b c d e Labib, Amir; Winters, Ryan (2024). "Complex Wound Management". StatPearls. Treasure Island, Florida: StatPearls Publishing. PMID 35015410. Retrieved 26 January 2024.
- ^ Jaman, Josip; Martić, Krešimir; Rasic, Nivez; Markulin, Helena; Haberle, Sara (December 2021). "Is the use of specific time cut-off or 'golden period' for primary closure of acute traumatic wounds evidence based? A systematic review". Croatian Medical Journal. 62 (6): 614–622. doi:10.3325/cmj.2021.62.614. ISSN 0353-9504. PMC 8771236. PMID 34981694.
- ^ Chhabra, Shruti; Chhabra, Naveen; Kaur, Avneet; Gupta, Niti (December 2017). "Wound Healing Concepts in Clinical Practice of OMFS". Journal of Maxillofacial and Oral Surgery. 16 (4): 403–423. doi:10.1007/s12663-016-0880-z. ISSN 0972-8279. PMC 5628060. PMID 29038623.
- ^ "Absorbable sutures in pediatric lacerations". BestBets. Archived from the original on 26 December 2008.
- ^ a b Cals JW, de Bont EG (October 2012). "Minor incised traumatic laceration". BMJ. 345: e6824. doi:10.1136/bmj.e6824. PMID 23092899. S2CID 32499629. Archived from the original on 5 November 2013.
- ^ Farion K, Osmond MH, Hartling L, Russell K, Klassen T, Crumley E, Wiebe N (2002). Farion KJ (ed.). "Tissue adhesives for traumatic lacerations in children and adults". The Cochrane Database of Systematic Reviews. 2002 (3): CD003326. doi:10.1002/14651858.CD003326. PMC 9006881. PMID 12137689.
- ^ Britto, Errol J.; Nezwek, Trevor A.; Popowicz, Patrycja; Robins, Marc (2024). "Wound Dressings". StatPearls. Treasure Island, Florida: StatPearls Publishing. PMID 29261956. Retrieved 26 January 2024.
- ^ Bhoyar, Surbhi D; Malhotra, Karan; Madke, Bhushan (April 2023). "Dressing materials: A comprehensive review". Journal of Cutaneous and Aesthetic Surgery. 16 (2): 81–89. doi:10.4103/JCAS.JCAS_163_22. ISSN 0974-2077. PMC 10405539. PMID 37554675.
- ^ Zaver, Vasudev; Kankanalu, Pradeep (2024). "Negative Pressure Wound Therapy". StatPearls. Treasure Island, Florida: StatPearls Publishing. PMID 35015413. Retrieved 26 January 2024.
- ^ a b Robert, N. (1 February 2017). "Negative pressure wound therapy in orthopaedic surgery". Orthopaedics & Traumatology: Surgery & Research. 2016 Instructional Course Lectures (SoFCOT). 103 (1, Supplement): S99–S103. doi:10.1016/j.otsr.2016.04.018. ISSN 1877-0568. PMID 28043851.
- ^ Bhedi, Amul; Saxena, Atul K.; Gadani, Ravi; Patel, Ritesh (April 2013). "Digital Photography and Transparency-Based Methods for Measuring Wound Surface Area". Indian Journal of Surgery. 75 (2): 111–114. doi:10.1007/s12262-012-0422-y. ISSN 0972-2068. PMC 3644165. PMID 24426404.
- ^ Jull AB, Cullum N, Dumville JC, Westby MJ, Deshpande S, Walker N (March 2015). "Honey as a topical treatment for wounds". The Cochrane Database of Systematic Reviews. 3 (3): CD005083. doi:10.1002/14651858.CD005083.pub4. PMC 9719456. PMID 25742878.
- ^ Garrett, Bernie; Riou, Marliss (20 March 2021). "A rapid evidence assessment of recent therapeutic touch research". Nursing Open. 8 (5): 2318–2330. doi:10.1002/nop2.841. ISSN 2054-1058. PMC 8363410. PMID 33742792.
- ^ Ghosh PK, Gaba A (2013). "Phyto-extracts in wound healing". Journal of Pharmacy & Pharmaceutical Sciences. 16 (5): 760–820. doi:10.18433/j3831v. PMID 24393557.
- ^ Bahramsoltani R, Farzaei MH, Rahimi R (September 2014). "Medicinal plants and their natural components as future drugs for the treatment of burn wounds: an integrative review". Archives of Dermatological Research. 306 (7): 601–617. doi:10.1007/s00403-014-1474-6. PMID 24895176. S2CID 23859340.
- ^ a b Saygin D, Tabib T, Bittar HE, Valenzi E, Sembrat J, Chan SY, et al. (1984). "Transcriptional profiling of lung cell populations in idiopathic pulmonary arterial hypertension". Pulmonary Circulation. 10 (1): 154–61. doi:10.2307/462158. JSTOR 462158. PMC 7052475. PMID 32166015.
- ^ Desiree May Oh, MD, Tania J. Phillips, MD (2006). "Sex Hormones and Wound Healing". Wounds. Archived from the original on 7 January 2013.
External links
- US based wound healing society
- Association for the Advancement of Wound Care AAWC
- European Wound Management Association – EWMA works to promote the advancement of education and research.