Potency and safety analysis of hemp-derived delta-9 products: The hemp vs. cannabis demarcation problem
Contents
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H28BrNO |
| Molar mass | 402.376 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 208 to 210 °C (406 to 410 °F) |
| |
| |
| (verify) | |
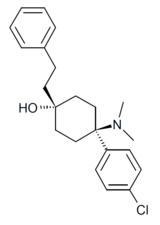
BDPC (systematic name 4-(4-bromophenyl)-4-(dimethylamino)-1-(2-phenylethyl)cyclohexanol; also known as bromadol) is a potent fully synthetic opioid with a distinctive arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s.[1] Initial studies estimated that it was around 10,000 times the potency of morphine in animal models.[2] However, later studies using more modern techniques assigned a value of 504 times the potency of morphine for the more active trans-isomer.[3] This drug was first seized along with three kilograms of acetylfentanyl in an April 25, 2013 police action in Montreal, Canada,[4] and has reportedly continued to be available on the designer drug market internationally.[5][6] Analogues where the para-bromine is replaced by chlorine or a methyl group retain similar activity, while the meta-hydroxyl derivative demonstrated robust antagonist activity.[7][8]


See also
References
- ^ US 4366172, Lednicer, Daniel, "4-Amino-cyclohexanols, their pharmaceutical compositions and methods of use", issued 1982-12-28, assigned to Upjohn Company
- ^ Lednicer D, VonVoigtlander PF (October 1979). "4-(p-Bromophenyl)-4-(dimethylamino)-1-phenethylcyclohexanol, an extremely potent representative of a new analgesic series". Journal of Medicinal Chemistry. 22 (10): 1157–8. doi:10.1021/jm00196a001. PMID 513062.
- ^ Liu ZH, Jin WQ, Dai QY, Chen XJ, Zhang HP, Chi ZQ (May 2003). "Opioid activity of C8813, a novel and potent opioid analgesic". Life Sciences. 73 (2): 233–41. doi:10.1016/S0024-3205(03)00263-7. PMID 12738037.
- ^ "Extremely potent painkiller hits Montreal black market". CBC News. May 13, 2013.
- ^ Sharma KK, Hales TG, Rao VJ, NicDaeid N, McKenzie C (January 2019). "The search for the "next" euphoric non-fentanil novel synthetic opioids on the illicit drugs market: current status and horizon scanning". Forensic Toxicology. 37 (1): 1–16. doi:10.1007/s11419-018-0454-5. PMC 6314991. PMID 30636980.
- ^ Vandeputte MM, Cannaert A, Stove CP (November 2020). "In vitro functional characterization of a panel of non-fentanyl opioid new psychoactive substances". Archives of Toxicology. 94 (11): 3819–3830. doi:10.1007/s00204-020-02855-7. hdl:1854/LU-8687070. PMID 32734307. S2CID 220881657.
- ^ Lednicer D, VonVoigtlander PF, Emmert DE (April 1981). "4-amino-4-arylcyclohexanones and their derivatives: a novel class of analgesics. 2. Modification of the carbonyl function". Journal of Medicinal Chemistry. 24 (4): 404–8. doi:10.1021/jm00136a010. PMID 7265128.
- ^ Lednicer D, Von Voigtlander PF, Emmert DE (March 1981). "4-aryl-4-aminocyclohexanones and their derivatives, a novel class of analgesics. 3. m-Hydroxyphenyl derivates". Journal of Medicinal Chemistry. 24 (3): 341–6. doi:10.1021/jm00135a019. PMID 7265120.

















