Type a search term to find related articles by LIMS subject matter experts gathered from the most trusted and dynamic collaboration tools in the laboratory informatics industry.
| Sickle cell disease | |
|---|---|
| Other names | Sickle cell disorder; drepanocytosis (dated) |
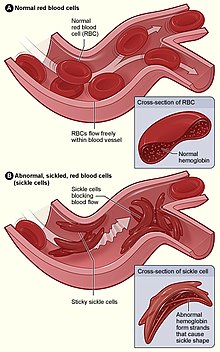 | |
| Figure (A) shows normal red blood cells flowing freely through a blood vessel. The inset shows a cross-section of a normal red blood cell with normal haemoglobin. Figure (B) shows abnormal, sickled red blood cells sticking at the branching point in a blood vessel. The inset image shows a cross-section of a sickle cell with long polymerized sickle haemoglobin (HbS) strands stretching and distorting the cell shape to look like a crescent moon. | |
| Specialty | Hematology, medical genetics |
| Symptoms | Attacks of pain, anemia, swelling in the hands and feet, bacterial infections, stroke[1] |
| Complications | Chronic pain, stroke, aseptic bone necrosis, gallstones, leg ulcers, priapism, pulmonary hypertension, vision problems, kidney problems[2] |
| Usual onset | 5–6 months of age[1] |
| Causes | Genetic, Homozygous mutation in the hemoglobin S gene.[3] |
| Diagnostic method | Blood test[4] |
| Treatment | Vaccination, antibiotics, high fluid intake, folic acid supplementation, pain medication, blood transfusions[5][6] |
| Prognosis | Life expectancy 40–60 years (developed world)[2] |
| Frequency | 7.7 million (2021)[7] |
| Deaths | 34,000 p.a. (a contributory factor to a further 376,000 p.a.)[7] |
Sickle cell disease (SCD), also simply called sickle cell, is a group of hemoglobin-related blood disorders that are typically inherited.[2] The most common type is known as sickle cell anemia.[2] Sickle cell anemia results in an abnormality in the oxygen-carrying protein haemoglobin found in red blood cells.[2] This leads to the red blood cells adopting an abnormal sickle-like shape under certain circumstances; with this shape, they are unable to deform as they pass through capillaries, causing blockages.[2] Problems in sickle cell disease typically begin around 5 to 6 months of age.[1] A number of health problems may develop, such as attacks of pain (known as a sickle cell crisis) in joints, anemia, swelling in the hands and feet, bacterial infections, dizziness[8] and stroke.[1] The probability of severe symptoms, including long-term pain, increases with age.[2] Without treatment, people with SCD rarely reach adulthood but with good healthcare, median life expectancy is between 58 and 66 years.[9][10] All the major organs are affected by sickle cell disease. The liver, heart, kidneys, gallbladder, eyes, bones, and joints also can suffer damage from the abnormal functions of the sickle cells, and their inability to flow through the small blood vessels correctly.[11]
Sickle cell disease occurs when a person inherits two abnormal copies of the β-globin gene that makes haemoglobin, one from each parent.[3] Several subtypes exist, depending on the exact mutation in each haemoglobin gene.[2] An attack can be set off by temperature changes, stress, dehydration, and high altitude.[1] A person with a single abnormal copy does not usually have symptoms and is said to have sickle cell trait.[3] Such people are also referred to as carriers.[5] Diagnosis is by a blood test, and some countries test all babies at birth for the disease.[4] Diagnosis is also possible during pregnancy.[4]
The care of people with sickle cell disease may include infection prevention with vaccination and antibiotics, high fluid intake, folic acid supplementation, and pain medication.[5][6] Other measures may include blood transfusion and the medication hydroxycarbamide (hydroxyurea).[6] In 2023, new gene therapies were approved involving the genetic modification and replacement of blood forming stem cells in the bone marrow.[12][13]
As of 2021, SCD is estimated to affect about 7.7 million people worldwide, directly causing 34,000 annual deaths and a contributory factor to a further 376,000 deaths.[7] About 80% of sickle cell disease cases are believed to occur in Sub-Saharan Africa.[14] It also occurs to a lesser degree in parts of India, Southern Europe, West Asia, North Africa and among people of African origin (sub-Saharan) living in other parts of the world.[15] The condition was first described in the medical literature by American physician James B. Herrick in 1910.[16][17] In 1949, its genetic transmission was determined by E. A. Beet and J. V. Neel.[17] In 1954, it was established that carriers of the abnormal gene are some degree protected against malaria.[17]

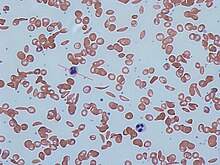

Signs of sickle cell disease usually begin in early childhood. The severity of symptoms can vary from person to person, as can the frequency of crisis events.[18][14] Sickle cell disease may lead to various acute and chronic complications, several of which have a high mortality rate.[19]
When SCD presents within the first year of life, the most common problem is an episode of pain and swelling in the child's hands and feet, known as dactylitis or "hand-foot syndrome." Pallor, jaundice, and fatigue can also be early signs due to anaemia resulting from sickle cell disease.[20]
Among children older than 2 years, the most frequent initial presentation is a painful event of generalized or variable type, and a slightly less common version appears as an event of acute chest pain. Dactylitis rarely or never occurs in children older than 2 years.[20][1]
Also termed "sickle cell crisis" or "sickling crisis", the vaso-occlusive crisis (VOC) manifests principally as extreme pain, most often affecting the chest, back, legs and/or arms.[21] The underlying cause is sickle-shaped red blood cells that obstruct capillaries and restrict blood flow to an organ, resulting in ischaemia, pain, necrosis, and often organ damage. The frequency, severity, and duration of these crises vary considerably. Painful crises are treated with hydration, analgesics, and blood transfusion; pain management requires opioid drug administration at regular intervals until the crisis has settled. For milder crises, a subgroup of patients manages on nonsteroidal anti-inflammatory drugs such as diclofenac or naproxen. For more severe crises, most patients require inpatient management for intravenous opioids; patient-controlled analgesia devices are commonly used in this setting. Vaso-occlusive crisis involving organs such as the penis[22] or lungs are considered an emergency and treated with red blood cell transfusions. Incentive spirometry, a technique to encourage deep breathing to minimise the development of atelectasis, is recommended.[23]
Although it is not always clear what sets off a VOC, anything which causes blood vessels to constrict is a potential trigger. This includes physical or mental stress, cold, and dehydration.[24] "After HbS deoxygenates in the capillaries, it takes some time (seconds) for HbS polymerization and the subsequent flexible-to-rigid transformation. If the transit time of RBC through the microvasculature is longer than the polymerization time, sickled RBC will lodge in the microvasculature."[25]
The spleen is especially prone to damage in SCD due to its role as a blood filter. A splenic sequestration crisis, also known as a spleen crisis, is a medical emergency that occurs when sickled red blood cells block the spleen's filter mechanism, causing the spleen to swell and fill with blood. The accumulation of red blood cells in the spleen results in a sudden drop in circulating hemoglobin and potentially life-threatening anemia. Symptoms include pain on the left side, swollen spleen (which can be detected by palpation), fatigue, dizziness, irritability, rapid heartbeat, or pale skin. It most commonly affects young children, the median age of first occurrence is 1.4 years. By the age of 5 years repeated instances of sequestration cause scarring and eventual atrophy of the spleen.[26][27][28]
Treatment is supportive, with blood transfusion if hemoglobin levels fall too low. Full or partial splenectomy may be necessary.[29] Long term consequences of a loss of spleen function are increased susceptibility to bacterial infections.[28]
Acute chest syndrome is caused by a VOC which affects the lungs, possibly triggered by infection or by emboli which have circulated from other organs. Symptoms include wheezing, chest pain, fever, pulmonary infiltrate (visible on x-ray), and hypoxemia. After sickling crisis (see above) it is the second-most common cause of hospitalization and it accounts for about 25% of deaths in patients with SCD. Most cases present with vaso-occlusive crises, and then develop acute chest syndrome.[30][31]
Aplastic crises are instances of an acute worsening of the patient's baseline anaemia, producing pale appearance, fast heart rate, and fatigue. This crisis is normally triggered by parvovirus B19, which directly affects production of red blood cells by invading the red cell precursors and multiplying in and destroying them.[32] Parvovirus infection [33]almost completely prevents red blood cell production for two to three days (red cell aplasia). In normal individuals, this is of little consequence, but the shortened red cell life of SCD patients results in an abrupt, life-threatening situation. Reticulocyte count drops dramatically during the disease (causing reticulocytopenia), red cell production lapses, and the rapid destruction of existing red cells leads to acute and severe anemia. This crisis takes four to seven days to resolve. Most patients can be managed supportively; some need a blood transfusion.[34]
Sickle cell anaemia can lead to various complications including:[35]

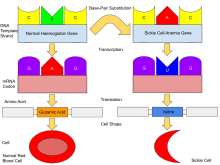
Hemoglobin is an oxygen-binding protein, found in erythrocytes, which transports oxygen from the lungs (or in the fetus, from the placenta) to the tissues. Each molecule of hemoglobin comprises 4 protein subunits, referred to as globins.[52] Normally, humans have:-
β-globin is encoded by the HBB gene on human chromosome 11; mutations in this gene produce variants of the protein which are implicated with abnormal hemoglobins. The mutation which causes sickle cell disease results in an abnormal hemoglobin known as hemoglobin S (HbS), which replaces HbA in adults.[18] The human genome contains a pair of genes for β-globin; in people with sickle cell disease, both genes are affected and the erythropoietic cells in the bone marrow will only create HbS. In people with sickle cell trait, only one gene is abnormal; erythropoiesis generates a mixture of normal HbA and sickle HbS. The person has very few if any symptoms of sickle cell disease but carries the gene and can pass it on to their children.[55]

Sickle cell disease has an autosomal recessive pattern of inheritance from parents.[56] Both copies of the affected gene must carry the same mutation (homozygous condition) for a person to be affected by an autosomal recessive disorder. An affected person usually has unaffected parents who each carry one mutated gene and one normal gene (heterozygous condition) and are referred to as genetic carriers; they may not have any symptoms.[57] When both parents have the sickle cell trait, any given child has a 25% chance of sickle cell disease; a 25% chance of no sickle cell alleles, and a 50% chance of the heterozygous condition (see diagram).[58]
There are several different haplotypes of the sickle cell gene mutation, indicating that it probably arose spontaneously in different geographic areas. The variants are known as Cameroon, Senegal, Benin, Bantu, and Saudi-Asian.[59] These are clinically important because some are associated with higher HbF levels, e.g., Senegal and Saudi-Asian variants, and tend to have milder disease.[60]

The gene defect is a single nucleotide mutation of the β-globin gene, which results in glutamate being substituted by valine at position 6 of the β-globin chain.[61] Hemoglobin S with this mutation is referred to as HbS, as opposed to the normal adult HbA. Under conditions of normal oxygen concentration this causes no apparent effects on the structure of haemoglobin or its ability to transport oxygen around the body. However, the deoxy form of HbS has an exposed hydrophobic patch which causes HbS molecules to join together to form long inflexible chains. Under conditions of low oxygen concentration in the bloodstream, such as exercise, stress, altitude or dehydration, HbS polymerization forms fibrous precipitates within the red blood cell.[62] In people homozygous for the sickle cell mutation, the presence of long-chain polymers of HbS distort the shape of the red blood cell from a smooth, doughnut-like shape to the sickle shape, making it fragile and susceptible to blocking or breaking within capillaries.[61]
In people heterozygous for HbS (carriers of sickle cell disease), the polymerisation problems are minor because the normal allele can produce half of the haemoglobin. Sickle cell carriers have symptoms only if they are deprived of oxygen (for example, at altitude) or while severely dehydrated.[63]

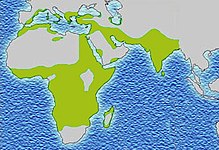
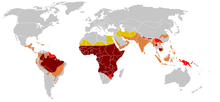
SCD is most prevalent in areas which have historically been associated with endemic malaria. The sickle cell trait provides a carrier with a survival advantage against malaria fatality over people with normal hemoglobin in regions where malaria is endemic.[64][65]
Infection with the malaria parasite affects asymptomatic carriers of the abnormal hemoglobin gene differently from patients with full SCD. Carriers (heterozygous for the gene) who catch malaria are less likely to suffer from severe symptoms than people with normal hemogolobin. SCD patients (homozygous for the gene) are similarly less likely to become infected with malaria; however once infected they are more likely to develop severe and life-threatening anemia.[66][67]
The impact of sickle cell anemia on malaria immunity illustrates some evolutionary trade-offs that have occurred because of endemic malaria. Although the shorter life expectancy for those with the homozygous condition would tend to disfavour the trait's survival, the trait is preserved in malaria-prone regions because of the benefits provided by the heterozygous form; an example of natural selection.[68]
Due to the adaptive advantage of the heterozygote, the disease is still prevalent, especially among people with recent ancestry in malaria-stricken areas, such as Africa, the Mediterranean, India, and the Middle East.[69] Malaria was historically endemic to southern Europe, but it was declared eradicated in the mid-20th century, with the exception of rare sporadic cases.[70]
The malaria parasite has a complex lifecycle and spends part of it in red blood cells. There are two mechanisms which protect sickle cell carriers from malaria. One is that the parasite is hindered from growing and reproducing in a carrier's red blood cells; another is that a carrier's red cells show signs of damage when infected, and are detected and destroyed as they pass through the spleen.[71][72]
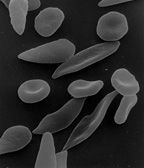
Under conditions of low oxygen concentration, HBS polymerises to form long strands within the red blood cell (RBC). These strands distort the shape of the cell and after a few seconds cause it to adopt an abnormal, inflexible sickle-like shape. This process reverses when oxygen concentration is raised and the cells resume their normal biconcave disc shape. If sickling takes place in the venous system, after blood has passed through the capillaries, it has no effect on the organs and the RBCs can unsickle when they become oxygenated in the lungs. Repeated switching between sickle and normal shapes damages the membrane of the RBC so that it eventually becomes permanently sickled.[73][74][75]
Normal red blood cells are quite elastic and have a biconcave disc shape, which allows the cells to deform to pass through capillaries. In sickle cell disease, low oxygen tension promotes red blood cell sickling and repeated episodes of sickling damage the cell membrane and decrease the cell's elasticity. These cells fail to return to normal shape when normal oxygen tension is restored. As a consequence, these rigid blood cells are unable to deform as they pass through narrow capillaries, leading to vessel occlusion and ischaemia.[25]
Cells which have become sickled are detected as they pass through the spleen and are destroyed. In young children with SCD, the accumulation of sickled cells in the spleen can result in splenic sequestration crisis. In this, the spleen becomes engorged with blood, depriving the general circulation of blood cells and leading to severe anemia. The spleen initially becomes noticeably swollen but the lack of a healthy blood flow through the organ culminates in scarring of the spleen tissues and eventually death of the organ, generally before the age of 5 years.[74][26]
The actual anaemia of the illness is caused by haemolysis, the destruction of the red cells, because of their shape. Although the bone marrow attempts to compensate by creating new red cells, it does not match the rate of destruction. Healthy red blood cells typically function for 90–120 days, but sickled cells only last 10–20 days.[76][74]
The rapid breakdown of RBC's in SCD results in the release of free heme into the bloodstream exceeding the capacity of the body's protective mechanisms. Although heme is an essential component of hemoglobin, it is also a potent oxidative molecule. Free heme is also an alarmin - a signal of tissue damage or infection, which triggers defensive responses in the body and increases the risk of inflammation and vaso-occlusive events.[77][78]
Checking for SCD begins during pregnancy, with a prenatal screening questionnaire which includes, among other things, a consideration of health issues in the child's parents and close relatives. A routine heel prick test, in which a small sample of blood is collected a few days after birth, is used to check conclusively for SCD as well as other inherited conditions.[79]
Where SCD is suspected, a number of tests can be used. Often a simpler, cheaper test is applied first with a more complex test such as DNA analysis used to confirm a positive result.[80]
Two tests which are specific for SCD:
Tests which can be used for SCD as well as for other hemoglobinopathies:
People who are known carriers of the disease or at risk of having a child with sickle cell anemia may undergo genetic counseling. Genetic counselors work with families to discuss the benefits, limitations, and logistics of genetic testing options as well as the potential impact of testing and test results on the individual.[84][85] During pregnancy, genetic testing can be done on either a blood sample from the fetus or a sample of amniotic fluid. During the first trimester of pregnancy, chorionic villus sampling (CVS) is also a technique used for SCD prenatal diagnosis.[86] Since taking a blood sample from a fetus has greater risks, the latter test is usually used. Neonatal screening sometimes referred to as newborn screening, provides not only a method of early detection for individuals with sickle cell disease but also allows for the identification of the groups of people who carry the sickle cell trait.[87] Genetic counselors can help individuals of colour and their families tackle the racial and ethnic disparities that exist in healthcare.[88]
Treatment involves a number of measures. While it has been historically recommended that people with sickle cell disease avoid exercise, regular exercise may benefit people.[89] Dehydration should be avoided.[90] A diet high in calcium is recommended[91] but the effectiveness of vitamin D supplementation remains uncertain.[92] L-glutamine use was supported by the FDA starting at the age of five, as it decreases complications.[93]
From birth to five years of age, penicillin daily, due to the immature immune system that makes them more prone to early childhood illnesses, is recommended.[94] Dietary supplementation of folic acid had been previously recommended by the WHO.[5] A 2016 Cochrane review of its use found "the effect of supplementation on anaemia and any symptoms of anaemia remains unclear" due to a lack of medical evidence.[95]

The protective effect of sickle cell trait does not apply to people with sickle cell disease; in fact, they are more vulnerable to malaria, since the most common cause of painful crises in malarial countries is infection with malaria. People with sickle cell disease living in malarial countries should receive lifelong medication for prevention.[96]
Most people with sickle cell disease have intensely painful episodes called vaso-occlusive crises. However, the frequency, severity, and duration of these crises vary tremendously. Painful crises are treated symptomatically with pain medications; pain management requires opioid drug administration at regular intervals until the crisis has settled. For milder crises, a subgroup of patients manages on NSAIDs (such as diclofenac or naproxen). For more severe crises, most patients require inpatient management for intravenous opioids.[97]
Extra fluids, administered either orally or intravenously, are a routine part of treatment of vaso-occlusive crises but the evidence about the most effective route, amount and type of fluid replacement remains uncertain.[98]
In 2019, Crizanlizumab, a monoclonal antibody target towards p-selectin was approved in the United States to reduce the frequency of vaso-occlusive crisis in those 16 years and older.[99]
Transcranial Doppler ultrasound (TCD) can detect children with sickle cell that have a high risk for stroke. The ultrasound test detects blood vessels partially obstructed by sickle cells by measuring the rate of blood into the brain, as blood flow velocity is inversely related to arterial diameter, and consequently, high blood-flow velocity is correlated with narrowing of the arteries.[100] In 2002, the National Institute of Health (NIH) issued a statement recommending that children with sickle cell get the Transcranial Doppler ultrasound screen annually, and in 2014, a panel of experts convened by the NIH issued guidelines reiterating the same recommendation. One review of medical records, by hematologist Dr. Julie Kanter at the University of Alabama at Birmingham, showed that on average only 48.4 per cent of children in the USA with sickle cell get the recommended ultrasound test.[101]
A 1994 NIH study showed that children at risk for strokes who received blood transfusions had an annual stroke rate of less than 1 per cent, whereas those children who did not receive blood transfusions had a 10 per cent stroke rate per year. (Also see 1998 study in the New England Journal of Medicine.[100]) In addition to ultrasounds and blood transfusions, the inexpensive generic drug hydroxyurea can reduce the risk of irreversible organ and brain damage. Guidelines from NIH published in 2014, state that all children and adolescents should take hydroxyurea, as should adults with serious complications or three or more pain crises in a year.[101]
Management is similar to vaso-occlusive crisis, with the addition of antibiotics (usually a quinolone or macrolide, since cell wall-deficient ["atypical"] bacteria are thought to contribute to the syndrome),[102] oxygen supplementation for hypoxia, and close observation. In the absence of high quality evidence regarding the effectiveness of antibiotics for acute chest syndrome in people with sickle cell disease, there is no standard antibiotic treatment as of 2019.[103]
Should the pulmonary infiltrate worsen or the oxygen requirements increase, simple blood transfusion or exchange transfusion is indicated. The latter involves the exchange of a significant portion of the person's red cell mass for normal red cells, which decreases the level of haemoglobin S in the patient's blood. However, there is currently uncertain evidence about the possible benefits or harms of blood transfusion for acute chest syndrome in people with sickle cell disease.[104]
Hydroxyurea, also known as hydroxycarbamide, probably reduces the frequency of painful episodes and the risk of life-threatening illness or death but there is currently insufficient evidence regarding the risk of adverse effects.[105] Hydroxyurea may be more effective than transfusion and chelation combined in terms of pain, life-threatening illness and risk of death.[105]
It was the first approved drug for the treatment of sickle cell anaemia, and was shown to decrease the number and severity of attacks in 1995[106] and shown to possibly increase survival time in a study in 2003.[107] This is achieved, in part, by reactivating fetal haemoglobin production in place of the haemoglobin S that causes sickle cell anaemia. Hydroxyurea had previously been used as a chemotherapy agent, and some concern exists that long-term use may be harmful, but this risk is either absent or very small and the benefits likely outweigh the risks.[19][108]
Voxelotor was approved in the United States in 2019, to increase hemoglobin in people with SS disease.[109] However, following an increased risk of vaso-occlusive seizures and death observed in registries and clinical trials, the European Medicines Agency suspended marketing authorization for voxelotor in September 2024, following which the manufacturer, Pfizer, withdrew it from the market worldwide.[110][111]
When treating avascular necrosis of the bone in people with sickle cell disease, the aim of treatment is to reduce or stop the pain and maintain joint mobility.[40] Current treatment options include resting the joint, physical therapy, pain-relief medicine, joint-replacement surgery, or bone grafting.[40] High quality, randomized, controlled trials are needed to assess the most effective treatment option and determine if a combination of physical therapy and surgery is more effective than physical therapy alone.[112][113]
Psychological therapies such as patient education, cognitive therapy, behavioural therapy, and psychodynamic psychotherapy, that aim to complement current medical treatments, require further research to determine their effectiveness.[114]
Blood transfusions are often used in the management of sickle cell disease in acute cases and to prevent complications by decreasing the number of red blood cells (RBCs) that can sickle by adding normal red blood cells.[115] In children, preventive RBC transfusion therapy has been shown to reduce the risk of first stroke or silent stroke when transcranial Doppler ultrasonography shows abnormal cerebral blood flow.[6] In those who have sustained a prior stroke event, it also reduces the risk of recurrent stroke and additional silent strokes.[116][117]
Bone marrow transplants have proven effective in children; they are the only known cure for SCD.[118] However, bone marrow transplants are difficult to obtain because of the specific HLA typing necessary. Ideally, a close relative (allogeneic) would donate the bone marrow necessary for transplantation. The close relative needs to have to same blood type as the patient. Some gene therapies are under development that would alter the patient's own bone marrow stem cells ex vivo, which can then be transplanted back into the patient after chemotherapy eliminates the original unmodified cells.[119]
Hematopoietic Stem Cell Transplantation
Hematopoietic stem cell transplantation (HSCT) involves replacing the dysfunctional stem cells from a person with sickle cell disease with healthy cells from a well-matched donor.[120] Finding the ideal donor—typically a stock or someone almost matched—is essential to the process' success. Different types of donors may be suitable and include umbilical cord blood, human leukocyte antigen (HLA) matched relatives, HLA matched donors that are not related to the person being treated, haploidentical but underwent myeloblative procedure (chemotherapy to kill the bone marrow cells), or other combinations.[120]
HSCT is used to treat for people with severe sickle cell disease in large centres, however, the evidence underlying the how effective this procedure is given the risks is not clear.[121] There is no strong medical evidence to determine the risks and potential benefits related to treating people with sickle cell disease with hematopoietic stem cell transplantations.[121][122] Risks associated with HSCT can include graft-versus host disease, death, failure of the graft, other toxicity related to the transplant.[120]
In 2023, both exagamglogene autotemcel and lovotibeglogene autotemcel were approved for the treatment of sickle cell disease.[12][123] On October 24, 2024, after 44 days of treatment, Kendric Cromer, the first commercial case to receive gene therapy, was discharged from Children's National Hospital. He underwent the latter therapy, and according to the companies involved, dozens of other patients are currently receiving these treatment.[124]
About 90% of people survive to age 20, and close to 50% survive beyond age 50.[125] In 2001, according to one study performed in Jamaica, the estimated mean survival for people was 53 years for men and 58 years for women with homozygous SCD.[126] The life expectancy in much of the developing world is unknown.[127] In 1975, about 7.3% of people with SCD died before their 23rd birthday; while in 1989, 2.6% of people with SCD died by the age of 20.[128]: 348
The HbS gene can be found in every ethnic group.[129] The highest frequency of sickle cell disease is found in tropical regions, particularly sub-Saharan Africa, tribal regions of India, and the Middle East.[130] About 80% of sickle cell disease cases are believed to occur in Sub-Saharan Africa.[14] Migration of substantial populations from these high-prevalence areas to low-prevalence countries in Europe has dramatically increased in recent decades and in some European countries, sickle cell disease has now overtaken more familiar genetic conditions such as haemophilia and cystic fibrosis.[131] In 2015, it resulted in about 114,800 deaths.[132]
Sickle cell disease occurs more commonly among people whose ancestors lived in tropical and subtropical sub-Saharan regions where malaria is or was common. Where malaria is common, carrying a single sickle cell allele (trait) confers a heterozygote advantage; humans with one of the two alleles of sickle cell disease show less severe symptoms when infected with malaria.[133]
This condition is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.[18]
Three-quarters of sickle cell cases occur in Africa. A recent WHO report estimated that around 2% of newborns in Nigeria were affected by sickle cell anaemia, giving a total of 150,000 affected children born every year in Nigeria alone. The carrier frequency ranges between 10 and 40% across equatorial Africa, decreasing to 1–2% on the North African coast and <1% in South Africa.[134] Studies in Africa show a significant decrease in infant mortality rate, ages 2–16 months, because of the sickle cell trait. This happened in areas of predominant malarial cases.[135]
Uganda has the fifth-highest sickle cell disease burden in Africa.[136] One study indicates that 20 000 babies per year are born with sickle cell disease with the sickle cell trait at 13.3% and with disease 0.7%.[137]
| Country | Population(2020) | Subregion | % of Prevalence | Prevalence | Incidence |
| Angola | 32,866,272 | Middle Africa | 0.09375 | 3,081,213 | 14,869 |
| Cameroon | 26,545,863 | Middle Africa | 0.117 | 3,105,866 | 11,826 |
| DR Congo | 89,561,403 | Middle Africa | 0.1163333333 | 10,418,977 | 65,536 |
| Ghana | 31,072,940 | Western Africa | 0.09375 | 2,913,088 | 9,588 |
| Guinea | 13,132,795 | Western Africa | 0.139375 | 1,830,383 | 8,907 |
| Niger | 24,206,644 | Western Africa | 0.07025 | 1,700,517 | 8,756 |
| Nigeria | 206,139,589 | Western Africa | 0.1286666667 | 26,523,294 | 150,000 |
| Tanzania | 59,734,218 | Eastern Africa | 0.0545 | 3,255,515 | 19,585 |
| Uganda | 45,741,007 | Eastern Africa | 0.07025 | 3,213,306 | 17,936 |
| Zambia | 18,383,955 | Eastern Africa | 0.082 | 1,507,484 | 9,958 |
| Algeria | 43,851,044 | Northern Africa | 0.029 | 1,271,680 | 6,624 |
| Benin | 12,123,200 | Western Africa | 0.1286666667 | 1,559,852 | 8,125 |
| Botswana | 2,351,627 | Southern Africa | 0.029 | 68,197 | 355 |
| Burkina Faso | 20,903,273 | Western Africa | 0.07025 | 1,468,455 | 7,649 |
| Burundi | 11,890,784 | Eastern Africa | 0.023 | 273,488 | 1,425 |
| Cabo Verde | 555,987 | Western Africa | 0.023 | 12,788 | 67 |
| Central African Republic | 4,829,767 | Middle Africa | 0.082 | 396,041 | 2,063 |
| Chad | 16,425,864 | Middle Africa | 0.0585 | 960,913 | 5,005 |
| Comoros | 869,601 | Eastern Africa | 0.023 | 20,001 | 104 |
| Congo | 5,518,087 | Middle Africa | 0.1615 | 891,171 | 4,642 |
| Côte d'Ivoire | 26,378,274 | Western Africa | 0.07025 | 1,853,074 | 9,652 |
| Djibouti | 988,000 | Eastern Africa | 0.023 | 22,724 | 118 |
| Egypt | 102,334,404 | Northern Africa | 0.029 | 2,967,698 | 15,458 |
| Equatorial Guinea | 1,402,985 | Middle Africa | 0.181 | 253,940 | 1,323 |
| Eritrea | 3,546,421 | Eastern Africa | 0.023 | 81,568 | 425 |
| Eswatini | 1,160,164 | Southern Africa | 0.023 | 26,684 | 139 |
| Ethiopia | 114,963,588 | Eastern Africa | 0.029 | 3,333,944 | 17,366 |
| Gabon | 2,225,734 | Middle Africa | 0.181 | 402,858 | 2,098 |
| Gambia | 2,416,668 | Western Africa | 0.082 | 198,167 | 1,032 |
| Guinea-Bissau | 1,968,001 | Western Africa | 0.035 | 68,880 | 359 |
| Kenya | 53,771,296 | Eastern Africa | 0.04675 | 2,513,808 | 13,094 |
| Lesotho | 2,142,249 | Southern Africa | 0.023 | 49,272 | 257 |
| Liberia | 5,057,681 | Western Africa | 0.07025 | 355,302 | 1,851 |
| Libya | 6,871,292 | Northern Africa | 0.029 | 199,267 | 1,038 |
| Madagascar | 27,691,018 | Eastern Africa | 0.04675 | 1,294,555 | 6,743 |
| Malawi | 19,129,952 | Eastern Africa | 0.035 | 669,548 | 3,488 |
| Mali | 20,250,833 | Western Africa | 0.082 | 1,660,568 | 8,650 |
| Mauritania | 4,649,658 | Western Africa | 0.04675 | 217,372 | 1,132 |
| Mauritius | 1,271,768 | Eastern Africa | 0.023 | 29,251 | 152 |
| Morocco | 36,910,560 | Northern Africa | 0.029 | 1,070,406 | 5,576 |
| Mozambique | 31,255,435 | Eastern Africa | 0.035 | 1,093,940 | 5,698 |
| Namibia | 2,540,905 | Southern Africa | 0.03883333333 | 98,672 | 514 |
| Rwanda | 12,952,218 | Eastern Africa | 0.035 | 453,328 | 2,361 |
| São Tomé and Príncipe | 219,159 | Middle Africa | 0.181 | 39,668 | 207 |
| Senegal | 16,743,927 | Western Africa | 0.07025 | 1,176,261 | 6,127 |
| Seychelles | 98,347 | Eastern Africa | 0.023 | 2,262 | 12 |
| Sierra Leone | 7,976,983 | Western Africa | 0.1615 | 1,288,283 | 6,711 |
| Somalia | 15,893,222 | Eastern Africa | 0.029 | 460,903 | 2,401 |
| South Africa | 59,308,690 | Southern Africa | 0.029 | 1,719,952 | 8,959 |
| South Sudan | 11,193,725 | Eastern Africa | 0.04675 | 523,307 | 2,726 |
| Sudan | 43,849,260 | Northern Africa | 0.03883333333 | 1,702,813 | 8,870 |
| Togo | 8,278,724 | Western Africa | 0.09375 | 776,130 | 4,043 |
| Tunisia | 11,818,619 | Northern Africa | 0.023 | 271,828 | 1,416 |
| Zimbabwe | 14,862,924 | Eastern Africa | 0.035 | 520,202 | 2,710 |
| Total | 1,338,826,604 | Africa | 91,868,664 | 495,726 |
The number of people with the disease in the United States is about 100,000 (one in 3,300), mostly affecting Americans of sub-Saharan African descent.[138] In the United States, about one out of 365 African-American children and one in every 16,300 Hispanic-American children have sickle cell anaemia.[139] The life expectancy for men with SCD is approximately 42 years of age while women live approximately six years longer.[140] An additional 2 million are carriers of the sickle cell trait.[141] Most infants with SCD born in the United States are identified by routine neonatal screening. As of 2016 all 50 states include screening for sickle cell disease as part of their newborn screen.[142] The newborn's blood is sampled through a heel-prick and is sent to a lab for testing. The baby must have been eating for a minimum of 24 hours before the heel-prick test can be done. Some states also require a second blood test to be done when the baby is two weeks old to ensure the results.[143]
Sickle cell anemia is the most common genetic disorder among African Americans. Approximately 8% are carriers and 1 in 375 are born with the disease.[144] Patient advocates for sickle cell disease have complained that it gets less government and private research funding than similar rare diseases such as cystic fibrosis, with researcher Elliott Vichinsky saying this shows racial discrimination or the role of wealth in health care advocacy.[145] Overall, without considering race, approximately 1.5% of infants born in the United States are carriers of at least one copy of the mutant (disease-causing) gene.[146]

As a result of population growth in African-Caribbean regions of overseas France and immigration from North and sub-Saharan Africa to mainland France, sickle cell disease has become a major health problem in France.[147] SCD has become the most common genetic disease in the country, with an overall birth prevalence of one in 2,415 in metropolitan France, ahead of phenylketonuria (one in 10,862), congenital hypothyroidism (one in 3,132), congenital adrenal hyperplasia (one in 19,008) and cystic fibrosis (one in 5,014) for the same reference period.[148]
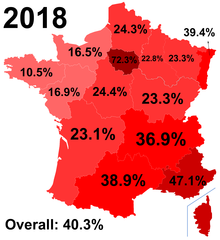
Since 2000, neonatal screening of SCD has been performed at the national level for all newborns defined as being "at-risk" for SCD based on ethnic origin (defined as those born to parents originating from sub-Saharan Africa, North Africa, the Mediterranean area (South Italy, Greece, and Turkey), the Arabic peninsula, the French overseas islands, and the Indian subcontinent).[149]
In the United Kingdom, between 12,000 and 15,000 people are thought to have sickle cell disease [150] with an estimated 250,000 carriers of the condition in England alone. As the number of carriers is only estimated, all newborn babies in the UK receive a routine blood test to screen for the condition.[151] Due to many adults in high-risk groups not knowing if they are carriers, pregnant women and both partners in a couple are offered screening so they can get counselling if they have the sickle cell trait.[152] In addition, blood donors from those in high-risk groups are also screened to confirm whether they are carriers and whether their blood filters properly.[153] Donors who are found to be carriers are informed and their blood, while often used for those of the same ethnic group, is not used for those with sickle cell disease who require a blood transfusion.[154]
In Saudi Arabia, about 4.2% of the population carry the sickle cell trait and 0.26% have sickle cell disease. The highest prevalence is in the Eastern province, where approximately 17% of the population carry the gene and 1.2% have sickle cell disease.[155] In 2005, Saudi Arabia introduced a mandatory premarital test including HB electrophoresis, which aimed to decrease the incidence of SCD and thalassemia.[156]
In Bahrain, a study published in 1998 that covered about 56,000 people in hospitals in Bahrain found that 2% of newborns have sickle cell disease, 18% of the surveyed people have the sickle cell trait, and 24% were carriers of the gene mutation causing the disease.[157] The country began screening of all pregnant women in 1992, and newborns started being tested if the mother was a carrier. In 2004, a law was passed requiring couples planning to marry to undergo free premarital counseling. These programs were accompanied by public education campaigns.[158]
Sickle cell disease is common in some ethnic groups of central India,[159] where the prevalence has ranged from 9.4 to 22.2% in endemic areas of Madhya Pradesh, Rajasthan, and Chhattisgarh.[160] It is also endemic among Tharu people of Nepal and India; however, they have a sevenfold lower rate of malaria despite living in a malaria infested zone.[161]
In Jamaica, 10% of the population carry the sickle cell gene, making it the most prevalent genetic disorder in the country.[162]
The first modern report of sickle cell disease may have been in 1846, where the autopsy of an executed runaway slave was discussed; the key finding was the absence of the spleen.[163][164] Reportedly, African slaves in the United States exhibited resistance to malaria, but were prone to leg ulcers.[164] The abnormal characteristics of the red blood cells, which later lent their name to the condition, was first described by Ernest E. Irons (1877–1959), intern to Chicago cardiologist and professor of medicine James B. Herrick (1861–1954), in 1910. Irons saw "peculiar elongated and sickle-shaped" cells in the blood of a man named Walter Clement Noel, a 20-year-old first-year dental student from Grenada. Noel had been admitted to the Chicago Presbyterian Hospital in December 1904 with anaemia.[16][165] Noel was readmitted several times over the next three years for "muscular rheumatism" and "bilious attacks" but completed his studies and returned to the capital of Grenada (St. George's) to practice dentistry. He died of pneumonia in 1916 and is buried in the Catholic cemetery at Sauteurs in the north of Grenada.[16][17] Shortly after the report by Herrick, another case appeared in the Virginia Medical Semi-Monthly with the same title, "Peculiar Elongated and Sickle-Shaped Red Blood Corpuscles in a Case of Severe Anemia."[166] This article is based on a patient admitted to the University of Virginia Hospital on 15 November 1910.[167] In the later description by Verne Mason in 1922, the name "sickle cell anemia" is first used.[17][168] Childhood problems related to sickle cells disease were not reported until the 1930s, despite the fact that this cannot have been uncommon in African-American populations.[164]
Memphis physician Lemuel Diggs, a prolific researcher into sickle cell disease, first introduced the distinction between sickle cell disease and trait in 1933, although until 1949, the genetic characteristics had not been elucidated by James V. Neel and E.A. Beet.[17] 1949 was the year when Linus Pauling described the unusual chemical behaviour of haemoglobin S, and attributed this to an abnormality in the molecule itself.[17][169] The molecular change in HbS was described in 1956 by Vernon Ingram.[170] The late 1940s and early 1950s saw further understanding in the link between malaria and sickle cell disease. In 1954, the introduction of haemoglobin electrophoresis allowed the discovery of particular subtypes, such as HbSC disease.[17]
Large-scale natural history studies and further intervention studies were introduced in the 1970s and 1980s, leading to widespread use of prophylaxis against pneumococcal infections amongst other interventions. Bill Cosby's Emmy-winning 1972 TV movie, To All My Friends on Shore, depicted the story of the parents of a child with sickle cell disease.[171] The 1990s had the development of hydroxycarbamide, and reports of cure through bone marrow transplantation appeared in 2007.[17]
Some old texts refer to it as drepanocytosis.[172]
Sickle cell disease is frequently contested as a disability.[173] Effective 15 September 2017, the U.S. Social Security Administration issued a Policy Interpretation Ruling providing background information on sickle cell disease and a description of how Social Security evaluates the disease during its adjudication process for disability claims.[174][175]
In the US, there are stigmas surrounding SCD that discourage people with SCD from receiving necessary care. These stigmas mainly affect people of African American and Latin American ancestries, according to the National Heart, Lung, and Blood Institute.[2] People with SCD experience the impact of stigmas of the disease on multiple aspects of life including social and psychological well-being. Studies have shown that those with SCD frequently feel as though they must keep their diagnosis a secret to avoid discrimination in the workplace and also among peers in relationships.[176] In the 1960s, the US government supported initiatives for workplace screening for genetic diseases in an attempt to be protective towards people with SCD. By having this screening, it was intended that employees would not be placed in environments that could potentially be harmful and trigger SCD.[177]
Uganda has the 5th highest sickle cell disease (SCD) burden in the world.[178] In Uganda, social stigma exists for those with sickle cell disease because of the lack of general knowledge of the disease. The general gap in knowledge surrounding sickle cell disease is noted among adolescents and young adults due to the culturally sanctioned secrecy about the disease.[178] While most people have heard generally about the disease, a large portion of the population is relatively misinformed about how SCD is diagnosed or inherited. Those who are informed about the disease learned about it from family or friends and not from health professionals. Failure to provide the public with information about sickle cell disease results in a population with a poor understanding of the causes of the disease, symptoms, and prevention techniques.[136] The differences, physically and socially, that arise in those with sickle cell disease, such as jaundice, stunted physical growth, and delayed sexual maturity, can also lead them to become targets of bullying, rejection, and stigma.[178]
The data compiled on sickle cell disease in Uganda has not been updated since the early 1970s. The deficiency of data is due to a lack of government research funds, even though Ugandans die daily from SCD.[179] Data shows that the trait frequency of sickle cell disease is 20% of the population in Uganda.[179] This means that 66 million people are at risk of having a child who has sickle cell disease.[179] It is also estimated that about 25,000 Ugandans are born each year with SCD and 80% of those people do not live past five years old.[179] SCD also contributes 25% to the child mortality rate in Uganda.[179] The Bamba people of Uganda, located in the southwest of the country, carry 45% of the gene which is the highest trait frequency recorded in the world.[179] The Sickle Cell Clinic in Mulago is only one sickle cell disease clinic in the country and on average sees 200 patients a day.[179]
The stigma around the disease is particularly bad in regions of the country that are not as affected. For example, Eastern Ugandans tend to be more knowledgeable of the disease than Western Ugandans, who are more likely to believe that sickle cell disease resulted as a punishment from God or witchcraft.[180] Other misconceptions about SCD include the belief that it is caused by environmental factors but, in reality, SCD is a genetic disease.[181] There have been efforts throughout Uganda to address the social misconceptions about the disease. In 2013, the Uganda Sickle Cell Rescue Foundation was established to spread awareness of sickle cell disease and combat the social stigma attached to the disease.[182] In addition to this organization's efforts, there is a need for the inclusion of sickle cell disease education in preexisting community health education programs in order to reduce the stigmatization of sickle cell disease in Uganda.[136]
The deeply rooted stigma of SCD from society causes families to often hide their family members' sick status for fear of being labeled, cursed, or left out of social events.[183] Sometimes in Uganda, when it is confirmed that a family member has sickle cell disease, intimate relationships with all members of the family are avoided.[183] The stigmatization and social isolation people with sickle cell disease tend to experience is often the consequence of popular misconceptions that people with SCD should not socialize with those free from the disease. This mentality robs people with SCD of the right to freely participate in community activities like everyone else[178] SCD-related stigma and social isolation in schools, especially, can make a life for young people living with sickle cell disease extremely difficult.[178] For school-aged children living with SCD, the stigma they face can lead to peer rejection.[178] Peer rejection involves the exclusion from social groups or gatherings. It often leads the excluded individual to experience emotional distress and may result in their academic underperformance, avoidance of school, and occupational failure later in life.[178] This social isolation is also likely to negatively impact people with SCD's self-esteem and overall quality of life.[178]
Mothers of children with sickle cell disease tend to receive disproportionate amounts of stigma from their peers and family members. These women will often be blamed for their child's diagnosis of SCD, especially if SCD is not present in earlier generations, due to the suspicion that the child's poor health may have been caused by the mother's failure to implement preventative health measures or promote a healthy environment for her child to thrive.[181] The reliance on theories related to environmental factors to place blame on the mother reflects many Ugandan's poor knowledge of how the disease is acquired as it is determined by genetics, not environment.[181] Mothers of children with sickle cell disease are also often left with very limited resources to safeguard their futures against the stigma of having SCD.[181] This lack of access to resources results from their subordinating roles within familial structures as well as the class disparities that hinder many mothers' ability to satisfy additional childcare costs and responsibilities.[181]
Women living with SCD who become pregnant often face extreme discrimination and discouragement in Uganda. These women are frequently branded by their peers as irresponsible for having a baby while living with sickle cell disease or even engaging in sex while living with SCD.Burden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): a cross-sectional study The criticism and judgement these women receive, not only from healthcare professionals but also from their families, often leaves them feeling alone, depressed, anxious, ashamed, and with very little social support.Burden of sickle cell trait and disease in the Uganda Sickle Surveillance Study (US3): a cross-sectional study - The Lancet Global Health Most pregnant women with SCD also go on to be single mothers as it is common for them to be left by their male partners who claim they were unaware of their partner's SCD status.Unprepared and Misinformed Parents of Children with Sickle Cell Disease: Time to Rethink Awareness Campaigns Not only does the abandonment experienced by these women cause emotional distress for them, but this low level of parental support can be linked to depressive symptoms and overall lower quality of life for the child once they are born.[184]
In 2021 many patients were found to be afraid to visit hospitals, such was the level of ignorance among staff, so purchased pain relief to treat themselves outside the NHS. They were often waiting a long time for pain relief, and sometimes suspected of "drugs-seeking" behaviour. Delays to treatment, failure to inform the hospital haematology team and poor pain management had caused deaths. Specialist haematology staff preferred to work in bigger, teaching hospitals, leading to shortages of expertise elsewhere.[185] In 2021, the NHS initiated its first new treatment in 20 years for Sickle Cell. This involved the use of Crizanlizumab, a drug given via transfusion drips, which reduces the number of visits to A&E by sufferers. The treatment can be accessed, via consultants, at any of ten new hubs set up around the country.[186] In the same year, however, an All-Party Parliamentary Group produced a report on Sickle Cell and Thalassaemia entitled 'No-one is listening'.[187]Partly in response to this, on 19 June 2022, World Sickle Cell Day, the NHS launched a campaign called "Can you tell it's sickle cell?". The campaign had twin aims. One was to increase awareness of the key signs and symptoms of the blood disorder so that people would be as alert to signs of a sickle cell crisis as they are to an imminent heart attack or stroke. The second aim was to set up a new training programme to help paramedics, Accident and Emergency staff, carers and the general public to care effectively for sufferers in crisis.[188]
Diseases such as sickle cell disease for which a person's normal phenotype or cell function may be restored in cells that have the disease by a normal copy of the gene that is mutated, may be a good candidate for gene therapy treatment. The risks and benefits related to gene therapy for sickle cell disease are not known.[189]
In 2001, sickle cell disease reportedly had been successfully treated in mice using gene therapy.[190][191] The researchers used a viral vector to make the mice—which have essentially the same defect that causes human sickle cell disease—express production of fetal haemoglobin (HbF), which an individual normally ceases to produce shortly after birth. In humans, using hydroxyurea to stimulate the production of HbF has been known to temporarily alleviate sickle cell disease symptoms. The researchers demonstrated that this gene therapy method is a more permanent way to increase therapeutic HbF production.[192]
Phase 1 clinical trials of gene therapy for sickle cell disease in humans were started in 2014. The clinical trials assessed the safety of lentiviral vector-modified bone marrow for adults with severe sickle cell disease.[193][194] A case report for the first person treated was published in March 2017, with a few more people being treated since then.[195][196]
Gene editing platforms like CRISPR/Cas9 have been used to correct the disease-causing mutation in hematopoietic stem cells taken from a person with the condition.[197] In July 2019 the gene-editing tool CRISPR was used to edit bone marrow cells from a person with SCD to boost fetal haemoglobin by inhibiting the BCL11A gene.[198][199] A number of researchers have considered the ethical implications of SCD being one of the first potential applications of CRISPR technology, given the historical abuses and neglect of the African American community by the medical field.[200]
In 2017, twelve clinical trials were focusing on gene therapy to treat sickle cell anemia. Of those 12 trials, four of them replaced the mutated HBB gene with a healthy one. Three trials used Mozobil, a medication used to treat types of cancer, to determine whether the increase of stem cells can be used for gene therapy. One trial focused on analyzing bone marrow samples from patients with sickle cell anemia. Another trial experimented with using umbilical cord blood from babies both with and without sickle cell anemia to develop gene therapy.[201]
In November 2023, a gene treatment using the CRISPR gene editing tool was approved by UK regulators for the treatment of sickle cell disease and also for the blood disorder transfusion-dependent beta thalassemia.[13][123]
While umbilical cord blood transplant can potentially cure the condition, a suitable donor is available in only 10% of people.[202] A cord blood transplant is not without serious risks and an estimated 7% of people die as a result of the cord blood transplant procedure and there is a risk of graft versus host disease.[202]
{{cite journal}}: CS1 maint: overridden setting (link)
twice-daily prophylactic penicillin beginning in early infancy and continuing through at least age 5
... teams report that two strategies for directly fixing malfunctioning blood cells have dramatically improved the health of a handful of people with these genetic diseases.
{{cite journal}}: CS1 maint: DOI inactive as of November 2024 (link)