Type a search term to find related articles by LIMS subject matter experts gathered from the most trusted and dynamic collaboration tools in the laboratory informatics industry.
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.
The potential energy stored in an explosive material may, for example, be:
Explosive materials may be categorized by the speed at which they expand. Materials that detonate (the front of the chemical reaction moves faster through the material than the speed of sound) are said to be "high explosives" and materials that deflagrate are said to be "low explosives". Explosives may also be categorized by their sensitivity. Sensitive materials that can be initiated by a relatively small amount of heat or pressure are primary explosives and materials that are relatively insensitive are secondary or tertiary explosives.
A wide variety of chemicals can explode; a smaller number are manufactured specifically for the purpose of being used as explosives. The remainder are too dangerous, sensitive, toxic, expensive, unstable, or prone to decomposition or degradation over short time spans.
In contrast, some materials are merely combustible or flammable if they burn without exploding.
The distinction, however, is not very clear. Certain materials—dusts, powders, gases, or volatile organic liquids—may be simply combustible or flammable under ordinary conditions, but become explosive in specific situations or forms, such as dispersed airborne clouds, or confinement or sudden release.

Early thermal weapons, such as Greek fire, have existed since ancient times. At its roots, the history of chemical explosives lies in the history of gunpowder.[1][2] During the Tang dynasty in the 9th century, Taoist Chinese alchemists were eagerly trying to find the elixir of immortality.[3] In the process, they stumbled upon the explosive invention of black powder made from coal, saltpeter, and sulfur in 1044. Gunpowder was the first form of chemical explosives and by 1161, the Chinese were using explosives for the first time in warfare.[4][5][6] The Chinese would incorporate explosives fired from bamboo or bronze tubes known as bamboo firecrackers. The Chinese also inserted live rats inside the bamboo firecrackers; when fired toward the enemy, the flaming rats created great psychological ramifications—scaring enemy soldiers away and causing cavalry units to go wild.[7]
The first useful explosive stronger than black powder was nitroglycerin, developed in 1847. Since nitroglycerin is a liquid and highly unstable, it was replaced by nitrocellulose, trinitrotoluene (TNT) in 1863, smokeless powder, dynamite in 1867 and gelignite (the latter two being sophisticated stabilized preparations of nitroglycerin rather than chemical alternatives, both invented by Alfred Nobel). World War I saw the adoption of TNT in artillery shells. World War II saw extensive use of new explosives .
In turn, these have largely been replaced by more powerful explosives such as C-4 and PETN. However, C-4 and PETN react with metal and catch fire easily, yet unlike TNT, C-4 and PETN are waterproof and malleable.[8]
The largest commercial application of explosives is mining. Whether the mine is on the surface or is buried underground, the detonation or deflagration of either a high or low explosive in a confined space can be used to liberate a fairly specific sub-volume of a brittle material (rock) in a much larger volume of the same or similar material. The mining industry tends to use nitrate-based explosives such as emulsions of fuel oil and ammonium nitrate solutions,[9] mixtures of ammonium nitrate prills (fertilizer pellets) and fuel oil (ANFO) and gelatinous suspensions or slurries[10] of ammonium nitrate and combustible fuels.
In materials science and engineering, explosives are used in cladding (explosion welding). A thin plate of some material is placed atop a thick layer of a different material, both layers typically of metal. Atop the thin layer is placed an explosive. At one end of the layer of explosive, the explosion is initiated. The two metallic layers are forced together at high speed and with great force. The explosion spreads from the initiation site throughout the explosive. Ideally, this produces a metallurgical bond between the two layers.
As the length of time the shock wave spends at any point is small, we can see mixing of the two metals and their surface chemistries, through some fraction of the depth, and they tend to be mixed in some way. It is possible that some fraction of the surface material from either layer eventually gets ejected when the end of material is reached. Hence, the mass of the now "welded" bilayer, may be less than the sum of the masses of the two initial layers.
There are applications[which?] where a shock wave, and electrostatics, can result in high velocity projectiles such as in an electrostatic particle accelerator.[11]
An explosion is a type of spontaneous chemical reaction that, once initiated, is driven by both a large exothermic change (great release of heat) and a large positive entropy change (great quantities of gases are released) in going from reactants to products, thereby constituting a thermodynamically favorable process in addition to one that propagates very rapidly. Thus, explosives are substances that contain a large amount of energy stored in chemical bonds. The energetic stability of the gaseous products and hence their generation comes from the formation of strongly bonded species like carbon monoxide, carbon dioxide, and nitrogen gas, which contain strong double and triple bonds having bond strengths of nearly 1 MJ/mole. Consequently, most commercial explosives are organic compounds containing –NO2, –ONO2 and –NHNO2 groups that, when detonated, release gases like the aforementioned (e.g., nitroglycerin, TNT, HMX, PETN, nitrocellulose).[12]
An explosive is classified as a low or high explosive according to its rate of combustion: low explosives burn rapidly (or deflagrate), while high explosives detonate. While these definitions are distinct, the problem of precisely measuring rapid decomposition makes practical classification of explosives difficult. For a reaction to be classified as a detonation as opposed to just a deflagration, the propagation of the reaction shockwave through the material being tested must be faster than the speed of sound through that material. The speed of sound through a liquid or solid material is usually orders of magnitude faster than the speed of sound through air or other gases.
Traditional explosives mechanics is based on the shock-sensitive rapid oxidation of carbon and hydrogen to carbon dioxide, carbon monoxide and water in the form of steam. Nitrates typically provide the required oxygen to burn the carbon and hydrogen fuel. High explosives tend to have the oxygen, carbon and hydrogen contained in one organic molecule, and less sensitive explosives like ANFO are combinations of fuel (carbon and hydrogen fuel oil) and ammonium nitrate. A sensitizer such as powdered aluminum may be added to an explosive to increase the energy of the detonation. Once detonated, the nitrogen portion of the explosive formulation emerges as nitrogen gas and toxic nitric oxides.
The chemical decomposition of an explosive may take years, days, hours, or a fraction of a second. The slower processes of decomposition take place in storage and are of interest only from a stability standpoint. Of more interest are the other two rapid forms besides decomposition: deflagration and detonation.
In deflagration, decomposition of the explosive material is propagated by a flame front which moves relatively slowly through the explosive material, i.e. at speeds less than the speed of sound within the substance (which is usually still higher than 340 m/s or 1,220 km/h in most liquid or solid materials)[13] in contrast to detonation, which occurs at speeds greater than the speed of sound. Deflagration is a characteristic of low explosive material.
This term is used to describe an explosive phenomenon whereby the decomposition is propagated by a shock wave traversing the explosive material at speeds greater than the speed of sound within the substance.[14] The shock front is capable of passing through the high explosive material at supersonic speeds — typically thousands of metres per second.
In addition to chemical explosives, there are a number of more exotic explosive materials, and exotic methods of causing explosions. Examples include nuclear explosives, and abruptly heating a substance to a plasma state with a high-intensity laser or electric arc.
Laser- and arc-heating are used in laser detonators, exploding-bridgewire detonators, and exploding foil initiators, where a shock wave and then detonation in conventional chemical explosive material is created by laser- or electric-arc heating. Laser and electric energy are not currently used in practice to generate most of the required energy, but only to initiate reactions.
To determine the suitability of an explosive substance for a particular use, its physical properties must first be known. The usefulness of an explosive can only be appreciated when the properties and the factors affecting them are fully understood. Some of the more important characteristics are listed below:
Sensitivity refers to the ease with which an explosive can be ignited or detonated, i.e., the amount and intensity of shock, friction, or heat that is required. When the term sensitivity is used, care must be taken to clarify what kind of sensitivity is under discussion. The relative sensitivity of a given explosive to impact may vary greatly from its sensitivity to friction or heat. Some of the test methods used to determine sensitivity relate to:
Specific explosives (usually but not always highly sensitive on one or more of the three above axes) may be idiosyncratically sensitive to such factors as pressure drop, acceleration, the presence of sharp edges or rough surfaces, incompatible materials, or even — in rare cases — nuclear or electromagnetic radiation. These factors present special hazards that may rule out any practical utility.
Sensitivity is an important consideration in selecting an explosive for a particular purpose. The explosive in an armor-piercing projectile must be relatively insensitive, or the shock of impact would cause it to detonate before it penetrated to the point desired. The explosive lenses around nuclear charges are also designed to be highly insensitive, to minimize the risk of accidental detonation.
The index of the capacity of an explosive to be initiated into detonation in a sustained manner. It is defined by the power of the detonator which is certain to prime the explosive to a sustained and continuous detonation. Reference is made to the Sellier-Bellot scale that consists of a series of 10 detonators, from n. 1 to n. 10, each of which corresponds to an increasing charge weight. In practice, most of the explosives on the market today are sensitive to an n. 8 detonator, where the charge corresponds to 2 grams of mercury fulminate.
The velocity with which the reaction process propagates in the mass of the explosive. Most commercial mining explosives have detonation velocities ranging from 1,800 m/s to 8,000 m/s. Today, velocity of detonation can be measured with accuracy. Together with density it is an important element influencing the yield of the energy transmitted for both atmospheric over-pressure and ground acceleration. By definition, a "low explosive", such as black powder, or smokeless gunpowder has a burn rate of 171–631 m/s.[15] In contrast, a "high explosive", whether a primary, such as detonating cord, or a secondary, such as TNT or C-4, has a significantly higher burn rate about 6900–8092 m/s.[16]
Stability is the ability of an explosive to be stored without deterioration.
The following factors affect the stability of an explosive:
The term power or performance as applied to an explosive refers to its ability to do work. In practice it is defined as the explosive's ability to accomplish what is intended in the way of energy delivery (i.e., fragment projection, air blast, high-velocity jet, underwater shock and bubble energy, etc.). Explosive power or performance is evaluated by a tailored series of tests to assess the material for its intended use. Of the tests listed below, cylinder expansion and air-blast tests are common to most testing programs, and the others support specific applications.
In addition to strength, explosives display a second characteristic, which is their shattering effect or brisance (from the French meaning to "break"). Brisance is important in determining the effectiveness of an explosion in fragmenting shells, bomb casings, and grenades. The rapidity with which an explosive reaches its peak pressure (power) is a measure of its brisance. Brisance values are primarily employed in France and Russia.
The sand crush test is commonly employed to determine the relative brisance in comparison to TNT. No test is capable of directly comparing the explosive properties of two or more compounds; it is important to examine the data from several such tests (sand crush, trauzl, and so forth) in order to gauge relative brisance. True values for comparison require field experiments.
Density of loading refers to the mass of an explosive per unit volume. Several methods of loading are available, including pellet loading, cast loading, and press loading, the choice being determined by the characteristics of the explosive. Dependent upon the method employed, an average density of the loaded charge can be obtained that is within 80–99% of the theoretical maximum density of the explosive. High load density can reduce sensitivity by making the mass more resistant to internal friction. However, if density is increased to the extent that individual crystals are crushed, the explosive may become more sensitive. Increased load density also permits the use of more explosive, thereby increasing the power of the warhead. It is possible to compress an explosive beyond a point of sensitivity, known also as dead-pressing, in which the material is no longer capable of being reliably initiated, if at all.[citation needed]
Volatility is the readiness with which a substance vaporizes. Excessive volatility often results in the development of pressure within rounds of ammunition and separation of mixtures into their constituents. Volatility affects the chemical composition of the explosive such that a marked reduction in stability may occur, which results in an increase in the danger of handling.
The introduction of water into an explosive is highly undesirable since it reduces the sensitivity, strength, and velocity of detonation of the explosive. Hygroscopicity is a measure of a material's moisture-absorbing tendencies. Moisture affects explosives adversely by acting as an inert material that absorbs heat when vaporized, and by acting as a solvent medium that can cause undesired chemical reactions. Sensitivity, strength, and velocity of detonation are reduced by inert materials that reduce the continuity of the explosive mass. When the moisture content evaporates during detonation, cooling occurs, which reduces the temperature of reaction. Stability is also affected by the presence of moisture since moisture promotes decomposition of the explosive and, in addition, causes corrosion of the explosive's metal container.
Explosives considerably differ from one another as to their behavior in the presence of water. Gelatin dynamites containing nitroglycerine have a degree of water resistance. Explosives based on ammonium nitrate have little or no water resistance as ammonium nitrate is highly soluble in water and is hygroscopic.
Many explosives are toxic to some extent. Manufacturing inputs can also be organic compounds or hazardous materials that require special handling due to risks (such as carcinogens). The decomposition products, residual solids, or gases of some explosives can be toxic, whereas others are harmless, such as carbon dioxide and water.
Examples of harmful by-products are:
"Green explosives" seek to reduce environment and health impacts. An example of such is the lead-free primary explosive copper(I) 5-nitrotetrazolate, an alternative to lead azide.[17]
Explosive material may be incorporated in the explosive train of a device or system. An example is a pyrotechnic lead igniting a booster, which causes the main charge to detonate.
The most widely used explosives are condensed liquids or solids converted to gaseous products by explosive chemical reactions and the energy released by those reactions. The gaseous products of complete reaction are typically carbon dioxide, steam, and nitrogen.[18] Gaseous volumes computed by the ideal gas law tend to be too large at high pressures characteristic of explosions.[19] Ultimate volume expansion may be estimated at three orders of magnitude, or one liter per gram of explosive. Explosives with an oxygen deficit will generate soot or gases like carbon monoxide and hydrogen, which may react with surrounding materials such as atmospheric oxygen.[18] Attempts to obtain more precise volume estimates must consider the possibility of such side reactions, condensation of steam, and aqueous solubility of gases like carbon dioxide.[20]
Oxygen balance is an expression that is used to indicate the degree to which an explosive can be oxidized. If an explosive molecule contains just enough oxygen to convert all of its carbon to carbon dioxide, all of its hydrogen to water, and all of its metal to metal oxide with no excess, the molecule is said to have a zero oxygen balance. The molecule is said to have a positive oxygen balance if it contains more oxygen than is needed and a negative oxygen balance if it contains less oxygen than is needed.[21] The sensitivity, strength, and brisance of an explosive are all somewhat dependent upon oxygen balance and tend to approach their maxima as oxygen balance approaches zero.
A chemical explosive may consist of either a chemically pure compound, such as nitroglycerin, or a mixture of a fuel and an oxidizer, such as black powder or grain dust and air.
Some chemical compounds are unstable in that, when shocked, they react, possibly to the point of detonation. Each molecule of the compound dissociates into two or more new molecules (generally gases) with the release of energy.
The above compositions may describe most of the explosive material, but a practical explosive will often include small percentages of other substances. For example, dynamite is a mixture of highly sensitive nitroglycerin with sawdust, powdered silica, or most commonly diatomaceous earth, which act as stabilizers. Plastics and polymers may be added to bind powders of explosive compounds; waxes may be incorporated to make them safer to handle; aluminium powder may be introduced to increase total energy and blast effects. Explosive compounds are also often "alloyed": HMX or RDX powders may be mixed (typically by melt-casting) with TNT to form Octol or Cyclotol.
An oxidizer is a pure substance (molecule) that in a chemical reaction can contribute some atoms of one or more oxidizing elements, in which the fuel component of the explosive burns. On the simplest level, the oxidizer may itself be an oxidizing element, such as gaseous or liquid oxygen.
The availability and cost of explosives are determined by the availability of the raw materials and the cost, complexity, and safety of the manufacturing operations.
A primary explosive is an explosive that is extremely sensitive to stimuli such as impact, friction, heat, static electricity, or electromagnetic radiation. Some primary explosives are also known as contact explosives. A relatively small amount of energy is required for initiation. As a very general rule, primary explosives are considered to be those compounds that are more sensitive than PETN. As a practical measure, primary explosives are sufficiently sensitive that they can be reliably initiated with a blow from a hammer; however, PETN can also usually be initiated in this manner, so this is only a very broad guideline. Additionally, several compounds, such as nitrogen triiodide, are so sensitive that they cannot even be handled without detonating. Nitrogen triiodide is so sensitive that it can be reliably detonated by exposure to alpha radiation.[22][23]
Primary explosives are often used in detonators or to trigger larger charges of less sensitive secondary explosives. Primary explosives are commonly used in blasting caps and percussion caps to translate a physical shock signal. In other situations, different signals such as electrical or physical shock, or, in the case of laser detonation systems, light, are used to initiate an action, i.e., an explosion. A small quantity, usually milligrams, is sufficient to initiate a larger charge of explosive that is usually safer to handle.
Examples of primary high explosives are:
A secondary explosive is less sensitive than a primary explosive and requires substantially more energy to be initiated. Because they are less sensitive, they are usable in a wider variety of applications and are safer to handle and store. Secondary explosives are used in larger quantities in an explosive train and are usually initiated by a smaller quantity of a primary explosive.
Examples of secondary explosives include TNT and RDX.
Tertiary explosives, also called blasting agents, are so insensitive to shock that they cannot be reliably detonated by practical quantities of primary explosive, and instead require an intermediate explosive booster of secondary explosive. These are often used for safety and the typically lower costs of material and handling. The largest consumers are large-scale mining and construction operations.
Most tertiaries include a fuel and an oxidizer. ANFO can be a tertiary explosive if its reaction rate is slow.
Low explosives (or low-order explosives) are compounds wherein the rate of decomposition proceeds through the material at less than the speed of sound. The decomposition is propagated by a flame front (deflagration) which travels much more slowly through the explosive material than a shock wave of a high explosive. Under normal conditions, low explosives undergo deflagration at rates that vary from a few centimetres per second to approximately 0.4 kilometres per second (1,300 ft/s). It is possible for them to deflagrate very quickly, producing an effect similar to a detonation. This can happen under higher pressure (such as when gunpowder deflagrates inside the confined space of a bullet casing, accelerating the bullet to well beyond the speed of sound) or temperature.
A low explosive is usually a mixture of a combustible substance and an oxidant that decomposes rapidly (deflagration); however, they burn more slowly than a high explosive, which has an extremely fast burn rate.[27]
Low explosives are normally employed as propellants. Included in this group are petroleum products such as propane and gasoline, gunpowder (including smokeless powder), and light pyrotechnics, such as flares and fireworks, but can replace high explosives in certain applications, including in gas pressure blasting.[28]
High explosives (HE, or high-order explosives) are explosive materials that detonate, meaning that the explosive shock front passes through the material at a supersonic speed. High explosives detonate with explosive velocity of about 3–9 kilometres per second (9,800–29,500 ft/s). For instance, TNT has a detonation (burn) rate of approximately 6.9 km/s (22,600 feet per second), detonating cord of 6.7 km/s (22,000 feet per second), and C-4 about 8.0 km/s (26,000 feet per second). They are normally employed in mining, demolition, and military applications. The term high explosive is in contrast with the term low explosive, which explodes (deflagrates) at a lower rate.
High explosives can be divided into two explosives classes differentiated by sensitivity: primary explosive and secondary explosive. Although tertiary explosives (such as ANFO at 3,200 m/s) can technically meet the explosive velocity definition, they are not considered high explosives in regulatory contexts.
Countless high-explosive compounds are chemically possible, but commercially and militarily important ones have included NG, TNT, TNP, TNX, RDX, HMX, PETN, TATP, TATB, and HNS.
Explosives are often characterized by the physical form that the explosives are produced or used in. These use forms are commonly categorized as:[29]
Shipping labels and tags may include both United Nations and national markings.
United Nations markings include numbered Hazard Class and Division (HC/D) codes and alphabetic Compatibility Group codes. Though the two are related, they are separate and distinct. Any Compatibility Group designator can be assigned to any Hazard Class and Division. An example of this hybrid marking would be a consumer firework, which is labeled as 1.4G or 1.4S.
Examples of national markings would include United States Department of Transportation (U.S. DOT) codes.
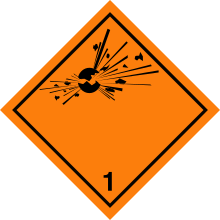
The UN GHS Hazard Class and Division (HC/D) is a numeric designator within a hazard class indicating the character, predominance of associated hazards, and potential for causing personnel casualties and property damage. It is an internationally accepted system that communicates using the minimum amount of markings the primary hazard associated with a substance.[30]
Listed below are the Divisions for Class 1 (Explosives):
To see an entire UNO Table, browse Paragraphs 3–8 and 3–9 of NAVSEA OP 5, Vol. 1, Chapter 3.
Compatibility Group codes are used to indicate storage compatibility for HC/D Class 1 (explosive) materials. Letters are used to designate 13 compatibility groups as follows.
The legality of possessing or using explosives varies by jurisdiction. Various countries around the world have enacted explosives law and require licenses to manufacture, distribute, store, use, possess explosives or ingredients.
In the Netherlands, the civil and commercial use of explosives is covered under the Wet explosieven voor civiel gebruik (explosives for civil use Act), in accordance with EU directive nr. 93/15/EEG[31] (Dutch). The illegal use of explosives is covered under the Wet Wapens en Munitie (Weapons and Munition Act)[32] (Dutch).
The new Explosives Regulations 2014 (ER 2014)[33] came into force on 1 October 2014 and defines "explosive" as:
"a) any explosive article or explosive substance which would —
(i) if packaged for transport, be classified in accordance with the United Nations Recommendations as falling within Class 1; or
(ii) be classified in accordance with the United Nations Recommendations as —
(aa) being unduly sensitive or so reactive as to be subject to spontaneous reaction and accordingly too dangerous to transport, and
(bb) falling within Class 1; or
(b) a desensitised explosive,
but it does not include an explosive substance produced as part of a manufacturing process which thereafter reprocesses it in order to produce a substance or preparation which is not an explosive substance"[33]
"Anyone who wishes to acquire and or keep relevant explosives needs to contact their local police explosives liaison officer. All explosives are relevant explosives apart from those listed under Schedule 2 of Explosives Regulations 2014."[34]
During World War I, numerous laws were created to regulate war related industries and increase security within the United States. In 1917, the 65th United States Congress created many laws, including the Espionage Act of 1917 and Explosives Act of 1917.
The Explosives Act of 1917 (session 1, chapter 83, 40 Stat. 385) was signed on 6 October 1917 and went into effect on 16 November 1917. The legal summary is "An Act to prohibit the manufacture, distribution, storage, use, and possession in time of war of explosives, providing regulations for the safe manufacture, distribution, storage, use, and possession of the same, and for other purposes". This was the first federal regulation of licensing explosives purchases. The act was deactivated after World War I ended.[35]
After the United States entered World War II, the Explosives Act of 1917 was reactivated. In 1947, the act was deactivated by President Truman.[36]
The Organized Crime Control Act of 1970 (Pub. L. 91–452) transferred many explosives regulations to the Bureau of Alcohol, Tobacco and Firearms (ATF) of the Department of Treasury. The bill became effective in 1971.[37]
Currently, regulations are governed by Title 18 of the United States Code and Title 27 of the Code of Federal Regulations:
Listed in alphabetical order: