Type a search term to find related articles by LIMS subject matter experts gathered from the most trusted and dynamic collaboration tools in the laboratory informatics industry.
 | |
| Clinical data | |
|---|---|
| Trade names | Ameile |
| Other names | Almonertinib; HS-10296 |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
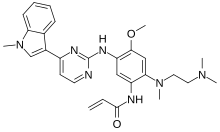
| Formula | C30H35N7O2 |
| Molar mass | 525.657 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Aumolertinib (trade name Ameile) is a pharmaceutical drug for the treatment of cancer.[1] It is an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI).[2]
In China, aumolertinib is approved for the treatment of patients with EGFR T790M mutation—positive non-small-cell lung cancer (NSCLC) who have progressed on or after other EGFR TKI therapy.[3][4]