Type a search term to find related articles by LIMS subject matter experts gathered from the most trusted and dynamic collaboration tools in the laboratory informatics industry.
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
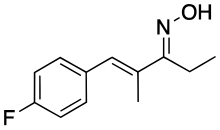
| Formula | C12H14FNO |
| Molar mass | 207.248 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
A-967079 is a drug which acts as a potent and selective antagonist for the TRPA1 receptor. It has analgesic and antiinflammatory effects and is used in scientific research, but has not been developed for medical use.[1][2][3][4][5]