Infrastructure tools to support an effective radiation oncology learning health system
Contents
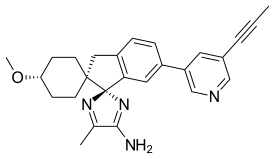
| |
| Names | |
|---|---|
| Systematic IUPAC name
(1,4-trans,1'R)-4-methoxy-5''-methyl-6'-[5-(prop-1-yn-1-yl)pyridin-3-yl]-3'H-dispiro[cyclohexane-1,2'-indene-1',2''-imidazol]-4''-amine | |
| Other names
AZD3293; LY3314814
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C26H28N4O | |
| Molar mass | 412.537 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lanabecestat (formerly known as AZD3293 or LY3314814) is an oral beta-secretase 1 cleaving enzyme (BACE) inhibitor. A BACE inhibitor in theory would prevent the buildup of beta-amyloid and may help slow or stop the progression of Alzheimer's disease.
In September 2014, AstraZeneca and Eli Lilly and Company announced an agreement to co-develop lanabecestat.[1] A pivotal Phase II/III clinical trial of lanabecestat started in late 2014 and is planned to recruit 2,200 patients and end in June 2019.[2] In April 2016 the company announced it would advance to phase 3 without modification.[3] AstraZeneca and Eli Lilly announced in August 2016 that they received FDA fast track designation for lanabecestat.[4] On June 12, 2018, Lilly and AstraZeneca announced that the two ongoing Phase III clinical trials for lanabecestat were not likely to meet their primary endpoint and that all current trials would be stopped due to futility.[5]
References
- ^ "AstraZeneca and Lilly announce alliance to develop and commercialise BACE inhibitor AZD3293 for Alzheimer's disease". www.astrazeneca.com. 16 Sep 2014. Retrieved 8 Oct 2014.
- ^ "AstraZeneca and Lilly move Alzheimer's drug into big trial". Reuters. December 2014.
- ^ Lilly and AstraZeneca Alzheimer's candidate advances; AstraZeneca earns $100M milestone. April 2016
- ^ "BRIEF-Lilly and Astrazeneca receive FDA fast track designation for AZD3293". www.reuters.com. 22 Aug 2016. Retrieved 25 Aug 2017.
- ^ "Update on Phase III Clinical Trials of lanabecestat". 12 June 2018. Retrieved 12 June 2018.

















