Infrastructure tools to support an effective radiation oncology learning health system
Contents
 | |
| Clinical data | |
|---|---|
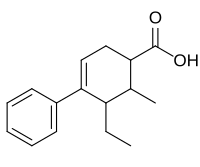
| Other names | Demethoxycarbestrol; NSC-86465; 2-methyl-3-ethyl-4-phenyl-δ4-cyclohexenecarboxylic acid |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H20O2 |
| Molar mass | 244.334 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Fenestrel (INN, USAN) (developmental code name ORF-3858) is a synthetic, nonsteroidal estrogen that was developed as a postcoital contraceptive in the 1960s but was never marketed.[1][2][3][4] Synthesized by Ortho Pharmaceutical in 1961 and studied extensively, it was coined the "morning-after-pill" or "postcoital antifertility agent".[5] Fenestrel is a seco analogue of doisynolic acid, and a member of the cyclohexenecarboxylic acid series of estrogens.[6][7]
See also
References
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 517–. ISBN 978-1-4757-2085-3.
- ^ Milne GW (8 May 2018). Drugs: Synonyms and Properties: Synonyms and Properties. Taylor & Francis. pp. 1407–. ISBN 978-1-351-78989-9.
- ^ Revaz C, Goldenberg B, Achtari H (January 1971). "[Critical study of new contraceptive methods]". Schweizerische Medizinische Wochenschrift (in French). 101 (3): 92–96. PMID 5544232.
- ^ Kunjappu MJ (June 2011). "Pioneering studies of the "morning-after" pill". The Yale Journal of Biology and Medicine. 84 (2): 109–111. PMC 3117403. PMID 21698041.
- ^ Hahn DW, McGuire JL (6 December 2012). "The Use of Pharmacological Agents to Study Implantation". In Glasser SR, Bullock DW (eds.). Cellular and Molecular Aspects of Implantation. Springer Science & Business Media. pp. 478–. ISBN 978-1-4613-3180-3.
- ^ Kirk RE, Othmer DF (1980). Encyclopedia of chemical technology. Wiley. p. 672. ISBN 978-0-471-02065-3.
- ^ Acta europaea fertilitatis. Piccin Medical Books. 1969.

















