Histopathology image classification: Highlighting the gap between manual analysis and AI automation
Contents
Appearance
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
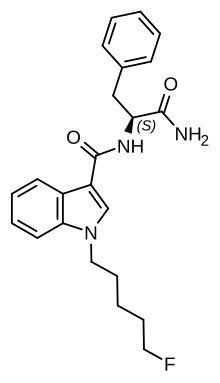
| Formula | C23H26FN3O2 |
| Molar mass | 395.478 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
PX-1 (also known as 5F-APP-PICA and SRF-30) is an indole-based synthetic cannabinoid that has been sold online as a designer drug.[1][2][3]
Legality
Sweden's public health agency suggested classifying PX-1 as hazardous substance on November 10, 2014.[4]
PX-1 is listed in the Fifth Schedule of the Misuse of Drugs Act (MDA) and therefore illegal in Singapore as of May 2015.[5]
See also
References
- ^ "PX 1". Cayman Chemical. Retrieved 15 July 2015.
- ^ Presley BC, Logan BK, Jansen-Varnum SA (March 2020). "Phase I metabolism of synthetic cannabinoid receptor agonist PX-1 (5F-APP-PICA) via incubation with human liver microsomes and UHPLC-HRMS". Biomedical Chromatography. 34 (3): e4786. doi:10.1002/bmc.4786. PMID 31863591. S2CID 209435138.
- ^ Dahm P, Thomas A, Rothschild MA, Thevis M, Mercer-Chalmers-Bender K (July 2022). "Phase I-metabolism studies of the synthetic cannabinoids PX-1 and PX-2 using three different in vitro models". Forensic Toxicology. 40 (2): 244–262. doi:10.1007/s11419-021-00606-6. PMC 9715525. PMID 36454402. S2CID 245540105.
- ^ "Cannabinoider föreslås bli klassade som hälsofarlig vara" [Cannabinoids are proposed to be classified as dangerous to health] (in Swedish). Folkhälsomyndigheten. Retrieved 16 July 2015.
- ^ "Misuse of Drugs Act". Singapore Government. 30 April 2015. Retrieved 24 July 2015.

















