Histopathology image classification: Highlighting the gap between manual analysis and AI automation
Contents
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
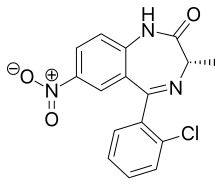
| Formula | C16H12ClN3O3 |
| Molar mass | 329.74 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Meclonazepam[1] ((S)-3-methylclonazepam) was discovered by a team at Hoffmann-La Roche in the 1970s and is a drug which is a benzodiazepine derivative similar in structure to clonazepam.[2] It has sedative and anxiolytic actions like those of other benzodiazepines,[3] and also has anti-parasitic effects against the parasitic worm Schistosoma mansoni.[4]
Meclonazepam was never used as medicine and instead appeared online as a designer drug.[5][6][7][8]
Legal Issues
United Kingdom
In the UK, meclonazepam has been classified as a Class C drug by the May 2017 amendment to The Misuse of Drugs Act 1971 along with several other designer benzodiazepine drugs.[9]
See also
References
- ^ US 4031078, Szente A, "Benzodiazepine derivatives", issued 21 June 1977, assigned to Hoffmann La Roche Inc.
- ^ The Lundbeck Institute. "Meclonazepam". Psychotropics. Lundbeck.
- ^ Ansseau M, Doumont A, Thiry D, von Frenckell R, Collard J (1985). "Initial study of methylclonazepam in generalized anxiety disorder. Evidence for greater power in the cross-over design" (PDF). Psychopharmacology. 87 (2): 130–135. doi:10.1007/bf00431795. PMID 3931136. S2CID 9776700.
- ^ O'Boyle C, Lambe R, Darragh A (1985). "Central effects in man of the novel schistosomicidal benzodiazepine meclonazepam". European Journal of Clinical Pharmacology. 29 (1): 105–108. doi:10.1007/bf00547377. PMID 4054198. S2CID 1150292.
- ^ Meyer MR, Bergstrand MP, Helander A, Beck O (May 2016). "Identification of main human urinary metabolites of the designer nitrobenzodiazepines clonazolam, meclonazepam, and nifoxipam by nano-liquid chromatography-high-resolution mass spectrometry for drug testing purposes". Analytical and Bioanalytical Chemistry. 408 (13): 3571–3591. doi:10.1007/s00216-016-9439-6. PMID 27071765. S2CID 25831532.
- ^ Pettersson Bergstrand M, Helander A, Hansson T, Beck O (April 2017). "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis. 9 (4): 640–645. doi:10.1002/dta.2003. PMID 27366870.
- ^ Manchester KR, Maskell PD, Waters L (March 2018). "Experimental versus theoretical log D7.4 , pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances". Drug Testing and Analysis. 10 (8): 1258–1269. doi:10.1002/dta.2387. PMID 29582576.
- ^ Manchester KR, Waters L, Haider S, Maskell PD (July 2022). "The blood-to-plasma ratio and predicted GABAA-binding affinity of designer benzodiazepines". Forensic Toxicology. 40 (2): 349–356. doi:10.1007/s11419-022-00616-y. PMC 9715504. PMID 36454409.
- ^ "The Misuse of Drugs Act 1971 (Amendment) Order 2017".

















