Histopathology image classification: Highlighting the gap between manual analysis and AI automation
Contents
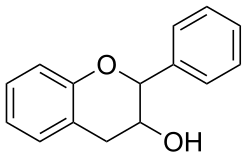
Flavan-3-ols (sometimes referred to as flavanols) are a subgroup of flavonoids. They are derivatives of flavans that possess a 2-phenyl-3,4-dihydro-2H-chromen-3-ol skeleton. Flavan-3-ols are structurally diverse and include a range of compounds, such as catechin, epicatechin gallate, epigallocatechin, epigallocatechin gallate, proanthocyanidins, theaflavins, thearubigins. They play a part in plant defense and are present in the majority of plants.[1]
Chemical structure
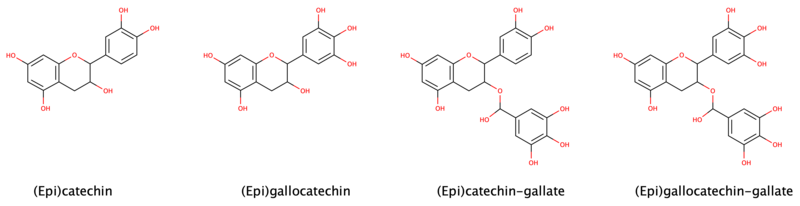
The single-molecule (monomer) catechin, or isomer epicatechin (see diagram), adds four hydroxyls to flavan-3-ol, making building blocks for concatenated polymers (proanthocyanidins) and higher order polymers (anthocyanidins).[2]
Flavan-3-ols possess two chiral carbons, meaning four diastereoisomers occur for each of them. They are distinguished from the yellow, ketone-containing flavonoids such as quercitin and rutin, which are called flavonols. Early use of the term bioflavonoid was imprecisely applied to include the flavanols, which are distinguished by absence of ketone(s). Catechin monomers, dimers, and trimers (oligomers) are colorless. Higher order polymers, anthocyanidins, exhibit deepening reds and become tannins.[2]
Catechin and epicatechin are epimers, with (–)-epicatechin and (+)-catechin being the most common optical isomers found in nature. Catechin was first isolated from the plant extract catechu, from which it derives its name. Heating catechin past its point of decomposition releases pyrocatechol (also called catechol), which explains the common origin of the names of these compounds.
Epigallocatechin and gallocatechin contain an additional phenolic hydroxyl group when compared to epicatechin and catechin, respectively, similar to the difference in pyrogallol compared to pyrocatechol.
Catechin gallates are gallic acid esters of the catechins; an example is epigallocatechin gallate, which is commonly the most abundant catechin in tea. Proanthocyanidins and thearubigins are oligomeric flavan-3-ols.
In contrast to many other flavonoids, flavan-3-ols do not generally exist as glycosides in plants.[3]
Biosynthesis of (–)-epicatechin
The flavonoids are products from a cinnamoyl-CoA starter unit, with chain extension using three molecules of malonyl-CoA. Reactions are catalyzed by a type III PKS enzyme. These enzyme do not use ACPSs, but instead employ coenzyme A esters and have a single active site to perform the necessary series of reactions, e.g. chain extension, condensation, and cyclization. Chain extension of 4-hydroxycinnamoyl-CoA with three molecules of malonyl-CoA gives initially a polyketide (Figure 1), which can be folded. These allow Claisen-like reactions to occur, generating aromatic rings.[4][5] Fluorescence-lifetime imaging microscopy (FLIM) can be used to detect flavanols in plant cells.[6]
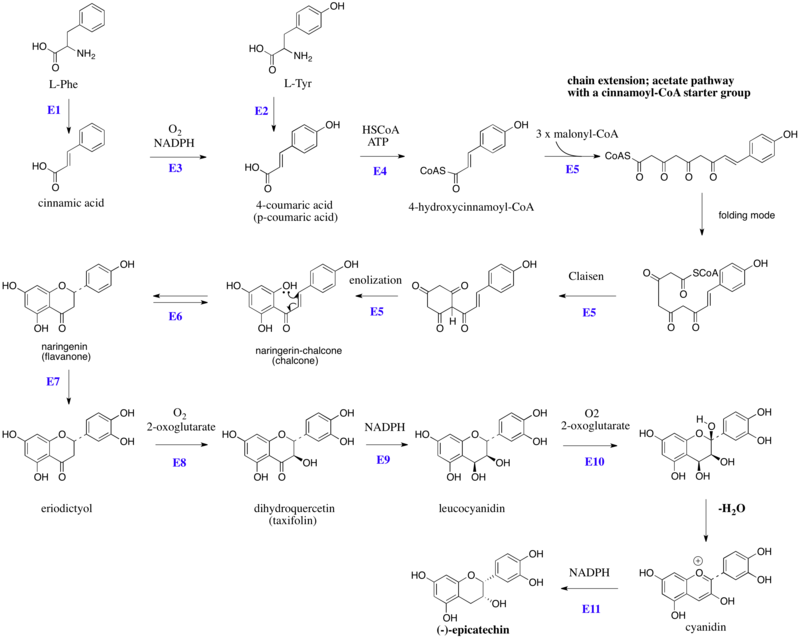
Figure 1:Schematic overview of the flavan-3-ol (–)-epicatechin biosynthesis in plants: Enzymes are indicated in blue, abbreviated as follows: E1, phenylalanine ammonia lyase (PAL), E2, tyrosine ammonia lyase (TAL), E3, cinnamate 4-hydroxylase, E4, 4-coumaroyl: CoA-ligase, E5, chalcone synthase (naringenin-chalcone synthase), E6, chalcone isomerase, E7, Flavonoid 3'-hydroxylase, E8, flavonone 3-hydroxylase, E9, dihydroflavanol 4-reductase, E10, anthocyanidin synthase (leucoanthocyanidin dioxygenase), E11, anthocyanidin reductase. HSCoA, Coenzyme A. L-Tyr, L-tyrosine, L-Phe, L-phenylalanine.
Aglycones
| Image | Name | Formula | Oligomers |
|---|---|---|---|
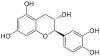 |
Catechin, C, (+)-Catechin | C15H14O6 | Procyanidins |
 |
Epicatechin, EC, (–)-Epicatechin (cis) | C15H14O6 | Procyanidins |
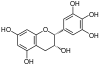 |
Epigallocatechin, EGC | C15H14O7 | Prodelphinidins |
 |
Epicatechin gallate, ECG | C22H18O10 | |
 |
Epigallocatechin gallate, EGCG, (–)-Epigallocatechin gallate |
C22H18O11 | |
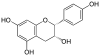 |
Epiafzelechin | C15H14O5 | |
| Fisetinidol | C15H14O5 | ||
| Guibourtinidol | C15H14O4 | Proguibourtinidins | |
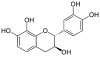 |
Mesquitol | C15H14O6 | |
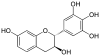 |
Robinetinidol | C15H14O6 | Prorobinetinidins |
Dietary sources
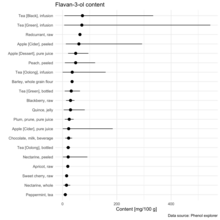
Flavan-3-ols are abundant in teas derived from the tea plant Camellia sinensis, as well as in some cocoas (made from the seeds of Theobroma cacao), although the content is affected considerably by processing, especially in chocolate.[8][9] Flavan-3-ols are also present in the human diet in fruits, in particular pome fruits, berries, vegetables, and wine.[10] Their content in food is variable and affected by various factors, such as cultivar, processing, and preparation.[11]
Bioavailability and metabolism
The bioavailability of flavan-3-ols depends on the food matrix, type of compound and their stereochemical configuration.[12] While monomeric flavan-3-ols are readily taken up, oligomeric forms are not absorbed.[12][13] Most data for human metabolism of flavan-3-ols are available for monomeric compounds, especially epiatechin. These compounds are taken up and metabolized upon uptake in the jejunum,[14] mainly by O-methylation and glucuronidation,[15] and then further metabolized by the liver. The colonic microbiome has also an important role in the metabolism of flavan-3-ols and they are catabolized to smaller compounds such as 5-(3′/4′-dihydroxyphenyl)-γ-valerolactones and hippuric acid.[16][17] Only flavan-3-ols with an intact (epi)catechin moiety can be metabolized into 5-(3′/4′-dihydroxyphenyl)-γ-valerolactones (image in Gallery).[18]
Possible adverse effects
As catechins in green tea extract can be hepatotoxic, Health Canada and EFSA have advised for caution,[19] recommending intake should not exceed 800 mg per day.[20]
Research
Research has shown that flavan-3-ols may affect vascular function, blood pressure, and blood lipids, with only minor effects demonstrated, as of 2019.[21][22] In 2015, the European Commission approved a health claim for cocoa solids containing 200 mg of flavanols, stating that such intake "may contribute to maintenance of vascular elasticity and normal blood flow".[23][24] As of 2022, food-based evidence indicates that intake of 400–600 mg per day of flavan-3-ols could have a small positive effect on cardiovascular biomarkers.[25]
Gallery
-
Schematic representation of the flavan-3-ol (−)-epicatechin metabolism in humans as a function of time post-oral intake. SREM: structurally related (−)-epicatechin metabolites. 5C-RFM: 5-carbon ring fission metabolites. 3/1C-RFM: 3- and 1-carbon-side chain ring fission metabolites. The structures of the most abundant (−)-epicatechin metabolites present in the systemic circulation and in urine are depicted.[17]
-
Flavan-3-ol precursors of the microbial metabolite 5-(3′/4′-dihydroxyphenyl)-γ-valerolactone (gVL). Only compounds with intact (epi)catechin moiety result in the formation of γVL by the intestinal microbiome. ECG, (−)-epicatechin-3-O-gallate; EGCG, Epigallocatechin gallate; EGC, Epigallocatechin.[18]
References
- ^ Ullah C, Unsicker SB, Fellenberg C, Constabel CP, Schmidt A, Gershenzon J, Hammerbacher A (December 2017). "Flavan-3-ols Are an Effective Chemical Defense against Rust Infection". Plant Physiology. 175 (4): 1560–1578. doi:10.1104/pp.17.00842. PMC 5717727. PMID 29070515.
- ^ a b Schwitters B, Masquelier J (1995). OPC in Practice (3rd ed.). OCLC 45289285.
- ^ Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A (May 2013). "Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases". Antioxidants & Redox Signaling. 18 (14): 1818–1892. doi:10.1089/ars.2012.4581. PMC 3619154. PMID 22794138.
- ^ Dewick PM (2009). Medicinal Natural Products: a biosynthetic approach. John Wiley & Sons. p. 168. ISBN 978-0-471-49641-0.
- ^ Winkel-Shirley B (June 2001). "Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology". Plant Physiology. 126 (2): 485–493. doi:10.1104/pp.126.2.485. PMC 1540115. PMID 11402179.
- ^ Mueller-Harvey I, Feucht W, Polster J, Trnková L, Burgos P, Parker AW, Botchway SW (March 2012). "Two-photon excitation with pico-second fluorescence lifetime imaging to detect nuclear association of flavanols". Analytica Chimica Acta. 719: 68–75. doi:10.1016/j.aca.2011.12.068. PMID 22340533. S2CID 24094780.
- ^ "Database on polyphenol content in foods, v. 3.6". Phenol Explorer. 2016.
- ^ Hammerstone JF, Lazarus SA, Schmitz HH (August 2000). "Procyanidin content and variation in some commonly consumed foods". The Journal of Nutrition. 130 (8S Suppl): 2086S–2092S. doi:10.1093/jn/130.8.2086S. PMID 10917927.
- ^ Payne MJ, Hurst WJ, Miller KB, Rank C, Stuart DA (October 2010). "Impact of fermentation, drying, roasting, and Dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients". Journal of Agricultural and Food Chemistry. 58 (19): 10518–10527. doi:10.1021/jf102391q. PMID 20843086.
- ^ Mabrym H, Harborne JB, Mabry TJ (1975). The Flavonoids. London: Chapman and Hall. ISBN 978-0-412-11960-6.
- ^ Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (May 2004). "Polyphenols: food sources and bioavailability". The American Journal of Clinical Nutrition. 79 (5): 727–747. doi:10.1093/ajcn/79.5.727. PMID 15113710.
- ^ a b Del Río D, Rodríguez Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A (May 2013). "Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases". Antioxidants & Redox Signaling. 18 (14): 1818–1892. doi:10.1089/ars.2012.4581. PMC 3619154. PMID 22794138.
- ^ Rodríguez Mateos A, Weber T, Skene SS, Ottaviani JI, Crozier A, Kelm M, et al. (December 2018). "Assessing the respective contributions of dietary flavanol monomers and procyanidins in mediating cardiovascular effects in humans: randomized, controlled, double-masked intervention trial". The American Journal of Clinical Nutrition. 108 (6): 1229–1237. doi:10.1093/ajcn/nqy229. PMC 6290365. PMID 30358831.
- ^ Actis-Goretta L, Lévèques A, Rein M, Teml A, Schäfer C, Hofmann U, et al. (October 2013). "Intestinal absorption, metabolism, and excretion of (−)-epicatechin in healthy humans assessed by using an intestinal perfusion technique". The American Journal of Clinical Nutrition. 98 (4): 924–933. doi:10.3945/ajcn.113.065789. PMID 23864538.
- ^ Kuhnle G, Spencer JP, Schroeter H, Shenoy B, Debnam ES, Srai SK, et al. (October 2000). "Epicatechin and catechin are O-methylated and glucuronidated in the small intestine". Biochemical and Biophysical Research Communications. 277 (2): 507–512. doi:10.1006/bbrc.2000.3701. PMID 11032751.
- ^ Das NP (December 1971). "Studies on flavonoid metabolism. Absorption and metabolism of (+)-catechin in man". Biochemical Pharmacology. 20 (12): 3435–3445. doi:10.1016/0006-2952(71)90449-7. PMID 5132890.
- ^ a b Ottaviani JI, Borges G, Momma TY, et al. (July 2016). "The metabolome of [2-14C](−)-epicatechin in humans: implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives". Scientific Reports. 6 (1): 29034. Bibcode:2016NatSR...629034O. doi:10.1038/srep29034. PMC 4929566. PMID 27363516.
- ^ a b Ottaviani JI, Fong R, Kimball J, Ensunsa JL, Britten A, Lucarelli D, et al. (June 2018). "Evaluation at scale of microbiome-derived metabolites as biomarker of flavan-3-ol intake in epidemiological studies". Scientific Reports. 8 (1): 9859. Bibcode:2018NatSR...8.9859O. doi:10.1038/s41598-018-28333-w. PMC 6026136. PMID 29959422.
- ^ Health Canada (12 December 2017). "Summary Safety Review - Green tea extract-containing natural health products - Assessing the potential risk of liver injury (hepatotoxicity)". Health Canada, Government of Canada. Retrieved 2022-05-06.
- ^ Younes M, Aggett P, Aguilar F, Crebelli R, Dusemund B, Filipič M, et al. (April 2018). "Scientific opinion on the safety of green tea catechins". EFSA Journal. 16 (4): e05239. doi:10.2903/j.efsa.2018.5239. PMC 7009618. PMID 32625874.
- ^ Ried K, Fakler P, Stocks NP, et al. (Cochrane Hypertension Group) (April 2017). "Effect of cocoa on blood pressure". The Cochrane Database of Systematic Reviews. 4 (5): CD008893. doi:10.1002/14651858.CD008893.pub3. PMC 6478304. PMID 28439881.
- ^ Raman G, Avendano EE, Chen S, Wang J, Matson J, Gayer B, et al. (November 2019). "Dietary intakes of flavan-3-ols and cardiometabolic health: systematic review and meta-analysis of randomized trials and prospective cohort studies". The American Journal of Clinical Nutrition. 110 (5): 1067–1078. doi:10.1093/ajcn/nqz178. PMC 6821550. PMID 31504087.
- ^ "Article 13 (5): Cocoa flavanols; Search filters: Claim status - authorised; search - flavanols". European Commission, EU Register. 31 March 2015. Retrieved 8 September 2022.
- ^ "Scientific Opinion on the modification of the authorisation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/20061 following a request in accordance with Article 19 of Regulation (EC) No 1924/2006". EFSA Journal. 12 (5). 2014. doi:10.2903/j.efsa.2014.3654.
- ^ Crowe-White, Kristi M; Evans, Levi W; Kuhnle, Gunter G C; Milenkovic, Dragan; Stote, Kim; Wallace, Taylor; Handu, Deepa; Senkus, Katelyn E (3 October 2022). "Flavan-3-ols and cardiometabolic health: First ever dietary bioactive guideline". Advances in Nutrition. 13 (6): 2070–83. doi:10.1093/advances/nmac105. PMC 9776652. PMID 36190328.
External links
 Media related to Flavan-3-ols at Wikimedia Commons
Media related to Flavan-3-ols at Wikimedia Commons
















![Schematic representation of the flavan-3-ol (−)-epicatechin metabolism in humans as a function of time post-oral intake. SREM: structurally related (−)-epicatechin metabolites. 5C-RFM: 5-carbon ring fission metabolites. 3/1C-RFM: 3- and 1-carbon-side chain ring fission metabolites. The structures of the most abundant (−)-epicatechin metabolites present in the systemic circulation and in urine are depicted.[17]](https://upload.wikimedia.org/wikipedia/commons/thumb/2/21/Schematic_representation_of_%28%E2%88%92%29-epicatechin_metabolism_in_humans_as_a_function_of_time_post-oral_intake.jpg/112px-Schematic_representation_of_%28%E2%88%92%29-epicatechin_metabolism_in_humans_as_a_function_of_time_post-oral_intake.jpg)
![Flavan-3-ol precursors of the microbial metabolite 5-(3′/4′-dihydroxyphenyl)-γ-valerolactone (gVL). Only compounds with intact (epi)catechin moiety result in the formation of γVL by the intestinal microbiome. ECG, (−)-epicatechin-3-O-gallate; EGCG, Epigallocatechin gallate; EGC, Epigallocatechin.[18]](https://upload.wikimedia.org/wikipedia/commons/thumb/f/f6/Flavan-3-ol_precursors_of_the_microbial_metabolite_5-%283%E2%80%B2-4%E2%80%B2-dihydroxyphenyl%29-%CE%B3-valerolactone.jpg/120px-Flavan-3-ol_precursors_of_the_microbial_metabolite_5-%283%E2%80%B2-4%E2%80%B2-dihydroxyphenyl%29-%CE%B3-valerolactone.jpg)

