Effects of the storage conditions on the stability of natural and synthetic cannabis in biological matrices for forensic toxicology analysis: An update from the literature
Contents
Appearance
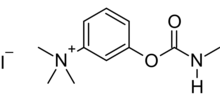
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
270 μg/kg (subcutaneous, mice)[1] 115 μg/kg (intravenous, mice)[1] 260 μg/kg (subcutaneous, rabbits)[1] |
LDLo (lowest published)
|
2.5 mg/kg (oral, mice)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
T-1152 is a quaternary carbamate anticholinesterase. It is synthesized by reaction of m-dimethylaminophenol with methyl isocyanate, followed by quaternization with methyl iodide.[3] Since T-1152 is toxic by ingestion, it was patented as a rodenticide in 1932.[2]
The chloride and methylsulfate salt of T-1152 is T-1690 (TL-1226) and AR-13, respectively.[1]
See also
References
- ^ a b c d Chemical Warfare Agents, and Related Chemical Problems. Parts I-II. 1958.
- ^ a b "Product for destroying animals".
- ^ Stedman, Edgar (1 January 1926). "Studies on the Relationship between Chemical Constitution and Physiological Action". Biochemical Journal. 20 (4): 719–734. doi:10.1042/bj0200719. PMC 1251776. PMID 16743713.

















