Effects of the storage conditions on the stability of natural and synthetic cannabis in biological matrices for forensic toxicology analysis: An update from the literature
Contents
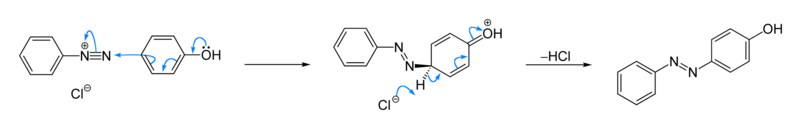
In organic chemistry, an azo coupling is an reaction between a diazonium compound (R−N≡N+) and another aromatic compound that produces an azo compound (R−N=N−R’). In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile, and the activated carbon (usually from an arene, which is called coupling agent), serves as a nucleophile. Classical coupling agents are phenols and naphthols.[1] Usually the diazonium reagent attacks at the para position of the coupling agent. When the para position is occupied, coupling occurs at a ortho position, albeit at a slower rate.
Uses of the reaction
Aromatic azo compounds tend to be brightly colored due to their extended conjugated systems. Many are useful dyes (see azo dye).[2] Important azo dyes include methyl red and pigment red 170.
Azo printing exploits this reaction as well. In this case, the diazonium ion is degraded by light, leaving a latent image in undegraded diazonium salt which is made to react with a phenol, producing a colored image: the blueprint.[3]
Prontosil, the first sulfa drug, was once produced by azo coupling. The azo compound is a prodrug that is activated in-vivo to produce the sufanilamide.
The reaction is also used in the Pauly reaction test to detect tyrosine or histidine residues in proteins.
Additionally, through the azo coupling reaction between the aromatic diazonium ion and aromatic amino acid residues, this reaction also be used to form or to modify proteins such as tRNA synthetase.[4]
Examples of azo C-coupling reactions
Illustrative is the reaction of phenol with benzenediazonium chloride to give a Solvent Yellow 7, a yellow-orange azo compound. The reaction is faster at high pH.[2] Many other azo dyes have been prepared by similarly. Several procedures have been described in detail.[5][6] [7]
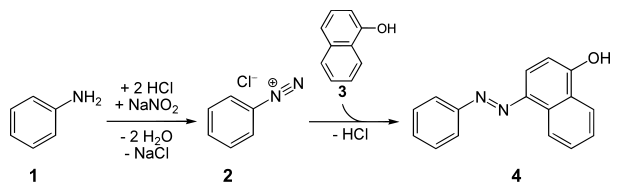
Naphthols are popular coupling agents. One example is the synthesis of the dye "organol brown" from aniline and 1-naphthol:
Similarly, β-naphthol couples with phenyldiazonium electrophile to produce an intense orange-red dye.
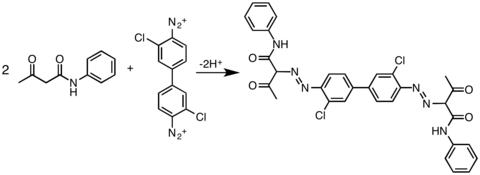
Besides activated aromatic coupling agents, other nucleophilic carbons could also be coupled with diazonium salt:
Examples of azo N-coupling reactions

In alkaline media, diazonium salt can react with most primary and secondary amines, which exist as a free base to produce triazene.[8] This chemical reaction is called azo N-coupling,[9] or the synthesis of azoamines.[10]
The dye called aniline yellow is produced by the reaction of aniline and a diazonium salt. In this case the C- and N-coupling compete.[2]
References
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ a b c Klaus Hunger; Peter Mischke; Wolfgang Rieper; Roderich Raue; Klaus Kunde; Aloys Engel (2005). "Azo Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245. ISBN 978-3527306732.
- ^ Pai, Damodar M.; Melnyk, Andrew R.; Weiss, David S.; Hann, Richard; Crooks, Walter; Pennington, Keith S.; Lee, Francis C.; Jaeger, C. Wayne; Titterington, Don R.; Lutz, Walter; Bräuninger, Arno; De Brabandere, Luc; Claes, Frans; De Keyzer, Rene; Janssens, Wilhelmus; Potts, Rod. "Imaging Technology, 2. Copying and Nonimpact Printing Processes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. pp. 1–53. doi:10.1002/14356007.o13_o08.pub2. ISBN 9783527306732.
- ^ Addy, Partha Sarathi; Erickson, Sarah B.; Italia, James S.; Chatterjee, Abhishek (2017-08-30). "A Chemoselective Rapid Azo-Coupling Reaction (CRACR) for Unclickable Bioconjugation". Journal of the American Chemical Society. 139 (34): 11670–11673. doi:10.1021/jacs.7b05125. ISSN 0002-7863. PMC 5861709. PMID 28787141.
- ^ J. L. Hartwell and Louis F. Fieser (1936). "Coupling of o-tolidine and Chicago acid". Organic Syntheses. 16: 12. doi:10.15227/orgsyn.016.0012.
- ^ H. T. Clarke and W. R. Kirner (1922). "Methyl red". Organic Syntheses. 2: 47. doi:10.15227/orgsyn.002.0047.
- ^ Conant, J. B.; Lutz, R. E.; Corson, B. B. (1923). "1,4-Aminonaphthol Hydrochloride". Organic Syntheses. 3: 7. doi:10.15227/orgsyn.003.0007.
- ^ Khazaei; et al. (2012). "azo amine coupling giving triazenes, and triazene's decomposition giving diazonium salt". Synlett. 23 (13): 1893–1896. doi:10.1055/s-0032-1316557. S2CID 196805424.
- ^ Wiley Subscription Services (2013). "Synthesis, characterization, and application of a triazene-base polymer". Journal of Applied Polymer Science. 129 (6): 3439–3446. doi:10.1002/app.39069.
- ^ Serge Ratton, Bernard Botannet (1981). "Preparation of aromatic azoamines by diazotization/coupling/rearrangement of aromatic amines". US Patent 4275003A.