Effects of the storage conditions on the stability of natural and synthetic cannabis in biological matrices for forensic toxicology analysis: An update from the literature
Contents
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
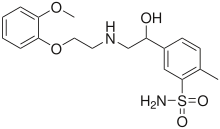
| Formula | C18H24N2O5S |
| Molar mass | 380.46 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Amosulalol (INN) is an antihypertensive drug. It has much higher affinity for α1-adrenergic receptors than for β-adrenergic receptors.[1] It is not approved for use in the United States.
Synthesis
Guaiacol (1) reacts with ethylene oxide to give 2-(2-methoxyphenoxy)ethanol (2). Halogenation with thionyl chloride converts the alcohol group to a chloride, (3), which is used to alkylate benzylamine (4) to give the secondary amine (5). This forms a tertiary amine (7) when combined with 5-bromoacetyl-2-methylbenzenesulfonamide (6). The reduction of the carbonyl group with sodium borohydride produces (8) and catalytic hydrogenation removes the benzyl group, yielding amosulalol.[2][3][4]
References
- ^ Sponer G, Bartsch W, Hooper RG (1990). "Drugs acting on multiple receptors: β-blockers with additional properties.". Pharmacology of antihypertensive therapeutics. Handbook of Experimental Pharmacology. Vol. 93. Berlin, Heidelberg: Springer. pp. 131–226 (183). doi:10.1007/978-3-642-74209-5_5. ISBN 978-3-642-74209-5.
- ^ US patent 4217305, Kazuo Imai, et al., "Phenylethanolamine derivatives", issued 1980-08-12, assigned to Yamanouchi Pharmaceutical Co. Ltd.
- ^ Arima, H.; Tamazawa, K.; Synthesis of 14C-labeled 5-[1-hydroxy-2-[2-(o-methoxyphenoxy)ethylamino]ethyl]-2-methylbenzenesulfonamide hydochloride (YM-09538). J Label Compd Radiopharm 1983, 20, 7, 803-811.
- ^ "Amosulalol". Pharmaceutical Substances. Thieme. Retrieved 2024-07-12.


















