Does cannabis extract obtained from cannabis flowers with maximum allowed residual level of aflatoxins and ochratoxin A have an impact on human safety and health?
Contents
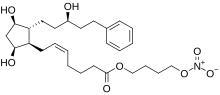 | |
| Clinical data | |
|---|---|
| Trade names | Vyzulta |
| Other names | BOL-303259-X |
| AHFS/Drugs.com | Monograph |
| License data | |
| Drug class | Prostaglandin analog |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.251.571 |
| Chemical and physical data | |
| Formula | C27H41NO8 |
| Molar mass | 507.624 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Latanoprostene bunod, sold under the brand name Vyzulta, is an ophthalmic medication used for the reduction of intraocular pressure in people with open-angle glaucoma or ocular hypertension.[2][3] It targets the trabecular meshwork directly.[3] It is a prostaglandin analog.[2]
The most common side effects include conjunctival (eye) redness, eye irritation, and eye discomfort (pain).[4] Latanoprostene bunod may cause the iris (colored part of the eye) to become darker in color.[4]
Latanoprostene bunod was approved for medical use in the United States in November 2017.[2][4][5]
Medical uses
Latanoprostene bunod is indicated for the reduction of intraocular pressure in people with open-angle glaucoma or ocular hypertension.[2]
History
The US Food and Drug Administration (FDA) approved latanoprostene bunod based on evidence from two clinical trials that enrolled 840 participants with open angle glaucoma or ocular hypertension.[4] The trials evaluated the benefits and side effects of latanoprostene bunod.[4] In each trial, participants were randomly assigned to receive either latanoprostene bunod or an approved drug timolol (ophthalmic solution) every day for three months.[4] Neither the participants nor the health care providers knew which treatment was being given until after the trials were completed.[4] The trials were conducted in the United States, the United Kingdom, Germany, Italy, Bulgaria, the Czech Republic, and Japan.[4]
References
- ^ "Regulatory Decision Summary for Vyzulta". Health Canada. 27 December 2018.
- ^ a b c d e "Vyzulta- latanoprostene bunod solution/ drops". DailyMed. 1 May 2019. Archived from the original on 7 June 2022. Retrieved 7 June 2022.
- ^ a b Hoy SM (May 2018). "Latanoprostene Bunod Ophthalmic Solution 0.024%: A Review in Open-Angle Glaucoma and Ocular Hypertension". Drugs. 78 (7): 773–780. doi:10.1007/s40265-018-0914-6. PMC 5976683. PMID 29761382.
- ^ a b c d e f g h "Vyzulta". U.S. Food and Drug Administration (FDA). 2 November 2017. Archived from the original on 11 July 2024. Retrieved 11 July 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Bausch + Lomb And Nicox Announce FDA Approval Of Vyzulta (Latanoprostene Bunod Ophthalmic Solution), 0.024%" (Press release). Valeant Pharmaceuticals. 2 November 2017. Archived from the original on 11 July 2024. Retrieved 11 July 2024 – via PR Newswire.

















