Does cannabis extract obtained from cannabis flowers with maximum allowed residual level of aflatoxins and ochratoxin A have an impact on human safety and health?
Contents
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
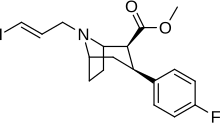
| Formula | C18H21FINO2 |
| Molar mass | 429.274 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Altropane (O-587, IACFT, 2β-carbomethoxy-3β-(4-fluorophenyl)-N-((E)-3-iodo-prop-2-enyl)tropane) is a phenyltropane derivative which acts as a potent dopamine reuptake inhibitor and long-acting stimulant drug. It has mainly been used as the 125I radiolabelled form for mapping the distribution of dopamine transporters in the brain,[1] and consequently this has led to its development as a potential diagnostic tool for early detection of Parkinson's disease.[2] It is also being investigated for potential use in the diagnosis and treatment of attention deficit hyperactivity disorder (ADHD).[3][4]
References
- ^ Elmaleh DR, Fischman AJ, Shoup TM, Byon C, Hanson RN, Liang AY, et al. (July 1996). "Preparation and biological evaluation of iodine-125-IACFT: a selective SPECT agent for imaging dopamine transporter sites". Journal of Nuclear Medicine. 37 (7): 1197–202. PMID 8965198.
- ^ Fischman AJ, Bonab AA, Babich JW, Palmer EP, Alpert NM, Elmaleh DR, et al. (June 1998). "Rapid detection of Parkinson's disease by SPECT with altropane: a selective ligand for dopamine transporters". Synapse. 29 (2): 128–41. doi:10.1002/(SICI)1098-2396(199806)29:2<128::AID-SYN4>3.0.CO;2-9. PMID 9593103. S2CID 30543189.
- ^ Cattabeni F (November 2002). "Altropane (Boston Life Science)". Current Opinion in Investigational Drugs. 3 (11): 1647–51. PMID 12476968.
- ^ Spencer TJ, Biederman J, Madras BK, Dougherty DD, Bonab AA, Livni E, et al. (November 2007). "Further evidence of dopamine transporter dysregulation in ADHD: a controlled PET imaging study using altropane". Biological Psychiatry. 62 (9): 1059–61. doi:10.1016/j.biopsych.2006.12.008. PMC 2715944. PMID 17511972.
















