February 8, 2023 - Astrix to Sponsor Lab of the Future 2023 in Boston
 The Lab of the Future Congress USA is back in Boston for 9-10 March 2023. Following the sell-out 2022 event, we have moved to the larger Renaissance Boston Waterfront to support the growth of the Congress.
Join us and hundreds of life science research leaders for the most innovative and best attended congress on the future of biopharma R&D. [Read More]
The Lab of the Future Congress USA is back in Boston for 9-10 March 2023. Following the sell-out 2022 event, we have moved to the larger Renaissance Boston Waterfront to support the growth of the Congress.
Join us and hundreds of life science research leaders for the most innovative and best attended congress on the future of biopharma R&D. [Read More]
January 31, 2023 - Astrix will be a corporate sponsor at the 2023 Scope Conferenc
 Astrix will be participating as a Corporate Sponsor of the SCOPE conference in Orlando Florida, February 6-9th, Booth 537. Join us and learn more about clinical operations and research [Read More]
Astrix will be participating as a Corporate Sponsor of the SCOPE conference in Orlando Florida, February 6-9th, Booth 537. Join us and learn more about clinical operations and research [Read More]
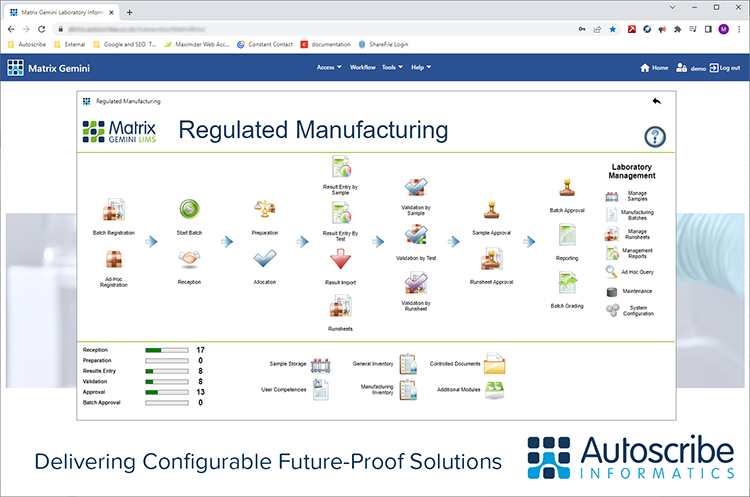
January 11, 2023 - Think a LIMS Is Not for Your Industry? – Think Again
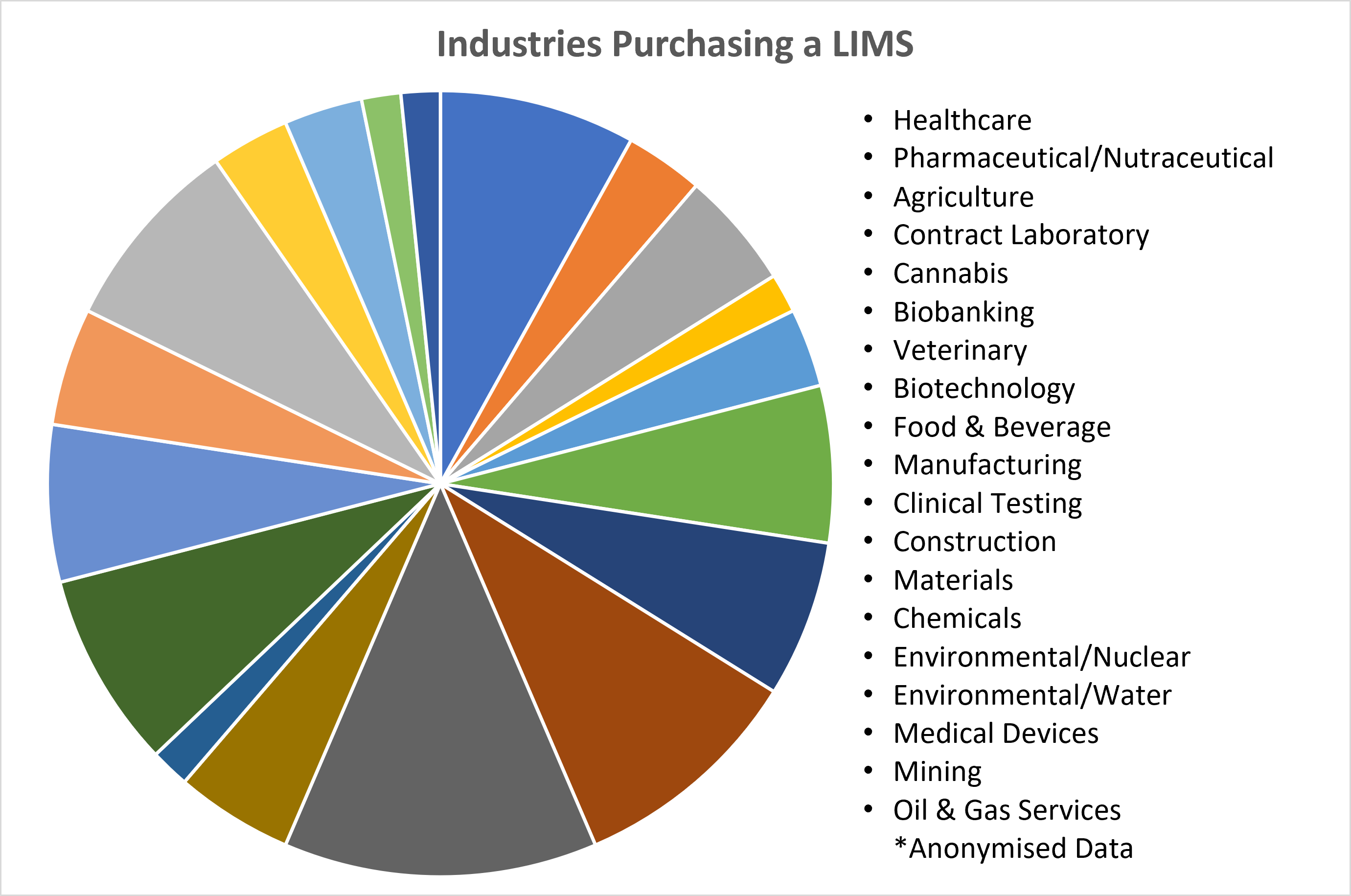 Recent data released by Autoscribe Informatics shows the breadth of industries purchasing a Laboratory Information Management System (LIMS); many for the first time. This market analysis from recent projects shows the cross-section of clients is moving well beyond what many think of as the LIMS core market of Healthcare, Medical Devices, and Pharmaceuticals. [Read More]
Recent data released by Autoscribe Informatics shows the breadth of industries purchasing a Laboratory Information Management System (LIMS); many for the first time. This market analysis from recent projects shows the cross-section of clients is moving well beyond what many think of as the LIMS core market of Healthcare, Medical Devices, and Pharmaceuticals. [Read More]
December 14, 2022 - Autoscribe Raises the Efficiency Bar with the Release of Matrix Gemini LIMS v6.6
 Autoscribe Informatics, the global leader in configurable Laboratory Information Management Systems (LIMS), today announced the release of Matrix Gemini version 6.6. The December 2022 release includes a number of enhancements designed to improve the user experience even further. [Read More]
Autoscribe Informatics, the global leader in configurable Laboratory Information Management Systems (LIMS), today announced the release of Matrix Gemini version 6.6. The December 2022 release includes a number of enhancements designed to improve the user experience even further. [Read More]
November 16, 2022 - CSols Inc. Validates New LIMS at Zosano Pharma
 CSols Inc. was recently contracted to validate WinLIMS for Zosano Pharma, a clinical-stage biopharmaceutical company that planned to transition away from paper record-keeping and take advantage of the increased efficiency and data accessibility offered by implementing a new laboratory information management system (LIMS). WinLIMS is a product of Quality System International Corporation (QSI). [Read More]
CSols Inc. was recently contracted to validate WinLIMS for Zosano Pharma, a clinical-stage biopharmaceutical company that planned to transition away from paper record-keeping and take advantage of the increased efficiency and data accessibility offered by implementing a new laboratory information management system (LIMS). WinLIMS is a product of Quality System International Corporation (QSI). [Read More]
November 16, 2022 - Agilent Announces AssayMAP Bravo Protein Sample Prep Workbench 4.0
 Agilent Technologies Inc. (NYSE: A) today announced the release of the AssayMAP Bravo Protein Sample Prep Workbench 4.0 software. This release adds 21 CFR Part 11 compliance-enabling features that allow AssayMAP Bravo-based automated sample preparation in workflows across the biopharma drug development process. [Read More]
Agilent Technologies Inc. (NYSE: A) today announced the release of the AssayMAP Bravo Protein Sample Prep Workbench 4.0 software. This release adds 21 CFR Part 11 compliance-enabling features that allow AssayMAP Bravo-based automated sample preparation in workflows across the biopharma drug development process. [Read More]
November 9, 2022 - QLIMS New LES Module – Interactive Digital SOPs
 We are pleased to announce the addition of our new LES (laboratory execution system) functionality to the QLIMS platform further enabling Lab 4.0 and the paperless, digital laboratory. [Read More]
We are pleased to announce the addition of our new LES (laboratory execution system) functionality to the QLIMS platform further enabling Lab 4.0 and the paperless, digital laboratory. [Read More]
November 9, 2022 - The Future of Clinisys: Comprehensive Laboratory Solutions
 Clinisys’ recent merger with Sunquest Information Systems & the subsequent acquisition of HORIZON Lab Systems, and ApolloLIMS has created the world’s largest laboratory informatics provider. Together now as Clinisys, we have over 3,000 customers in 34 countries, & our solutions power laboratories across the world that span healthcare, life sciences, environmental sciences, & public health. [Read More]
Clinisys’ recent merger with Sunquest Information Systems & the subsequent acquisition of HORIZON Lab Systems, and ApolloLIMS has created the world’s largest laboratory informatics provider. Together now as Clinisys, we have over 3,000 customers in 34 countries, & our solutions power laboratories across the world that span healthcare, life sciences, environmental sciences, & public health. [Read More]
October 19, 2022 - Autoscribe Demonstrates New LIMS Web Application at Lab Innovations
 Autoscribe Informatics will be demonstrating their new LIMS Web application at Lab Innovations 2022, the UK’s largest dedicated exhibition for laboratory professionals. Released in Matrix Gemini version 6.5 the new Matrix Gemini Web application is a major upgrade to the Web interface. It can be used in parallel with the original Web application allowing users to compare, test and validate as required. [Read More]
Autoscribe Informatics will be demonstrating their new LIMS Web application at Lab Innovations 2022, the UK’s largest dedicated exhibition for laboratory professionals. Released in Matrix Gemini version 6.5 the new Matrix Gemini Web application is a major upgrade to the Web interface. It can be used in parallel with the original Web application allowing users to compare, test and validate as required. [Read More]
October 12, 2022 - Introducing STARLIMS Technology Platform V12.4
 STARLIMS is pleased to announce the release of STARLIMS Technology Platform v12.4 (TP v12.4). This new release is available as an upgrade to all STARLIMS v10 – v12 customers. Upgrading the Technology Platform of your STARLIMS system is a quick and easy process that will bring new features, performance enhancements and other fixes to your system. [Read More]
STARLIMS is pleased to announce the release of STARLIMS Technology Platform v12.4 (TP v12.4). This new release is available as an upgrade to all STARLIMS v10 – v12 customers. Upgrading the Technology Platform of your STARLIMS system is a quick and easy process that will bring new features, performance enhancements and other fixes to your system. [Read More]
October 5, 2022 - Autoscribe Informatics Announces 3Q22 LIMS Starter Systems Release
October 5, 2022 - Clinisys completes combination of Sunquest, HORIZON and ApolloLIMS businesses
 Clinisys today announces it has completed the combination of Sunquest Information Systems, HORIZON Lab Systems, and Apollo LIMS under its singular brand. The completion marks a new chapter for Clinisys, expanding its global presence and breadth of expertise to enable healthier communities through the transformation of the modern laboratory. [Read More]
Clinisys today announces it has completed the combination of Sunquest Information Systems, HORIZON Lab Systems, and Apollo LIMS under its singular brand. The completion marks a new chapter for Clinisys, expanding its global presence and breadth of expertise to enable healthier communities through the transformation of the modern laboratory. [Read More]
September 20, 2022 - Astrix will be a Gold sponsor at the World Drug Safety Congress 2022
 Astrix will be a Gold Sponsor at this year’s World Drug Safety Congress (WDSC) event, October 4-5 in Boston, Massachusetts. Astrix will be at booth 32 and will be discussing services focused on pharmacovigilance. [Read More]
Astrix will be a Gold Sponsor at this year’s World Drug Safety Congress (WDSC) event, October 4-5 in Boston, Massachusetts. Astrix will be at booth 32 and will be discussing services focused on pharmacovigilance. [Read More]
September 7, 2022 - Agilent Collaborates with METTLER TOLEDO to Reduce Errors in Sample Preparation Workflows for Better Chromatographic Results
 Agilent Technologies Inc. announces that it is collaborating with METTLER TOLEDO to address one of the biggest concerns in any laboratory—error-prone sample preparation. This integrated solution allows the automatic and seamless transfer of weighing results and the associated metadata from METTLER TOLEDO LabX™ Balance software to Agilent OpenLab software. [Read More]
Agilent Technologies Inc. announces that it is collaborating with METTLER TOLEDO to address one of the biggest concerns in any laboratory—error-prone sample preparation. This integrated solution allows the automatic and seamless transfer of weighing results and the associated metadata from METTLER TOLEDO LabX™ Balance software to Agilent OpenLab software. [Read More]
August 31, 2022 - SampleVision™ v3.0—Access to Lab Results from Anywhere for FDA Regulated Environments
 LIMS Wizards, LLC, a global scientific software solutions provider, announces the launch of version 3.0 of SampleVision. The latest version offers full regulatory compliance for life sciences customers via audit trail and electronic signatures as well as cutting-edge features like geolocation services, dashboards, configurable reports, image capture and barcoding. [Read More]
LIMS Wizards, LLC, a global scientific software solutions provider, announces the launch of version 3.0 of SampleVision. The latest version offers full regulatory compliance for life sciences customers via audit trail and electronic signatures as well as cutting-edge features like geolocation services, dashboards, configurable reports, image capture and barcoding. [Read More]
August 31, 2022 - Thermo Fisher Scientific Launches CE-IVD (IVDD) Next-Generation Sequencing Test and Analysis Software
 Next-generation sequencing (NGS) is quickly becoming the platform of choice for tumor molecular profiling due to its ability to simultaneously report on multiple biomarkers. However, lengthy turnaround times can limit the clinical utility of these results. To meet the need for rapid genomic insights, Thermo Fisher Scientific today launched the CE-IVD (IVDD) Oncomine Dx Express Test and Oncomine Reporter Dx for use in clinical labs. [Read More]
Next-generation sequencing (NGS) is quickly becoming the platform of choice for tumor molecular profiling due to its ability to simultaneously report on multiple biomarkers. However, lengthy turnaround times can limit the clinical utility of these results. To meet the need for rapid genomic insights, Thermo Fisher Scientific today launched the CE-IVD (IVDD) Oncomine Dx Express Test and Oncomine Reporter Dx for use in clinical labs. [Read More]
August 24, 2022 - New LIMS Web Application Released by Autoscribe Informatics in Matrix Gemini v6.5
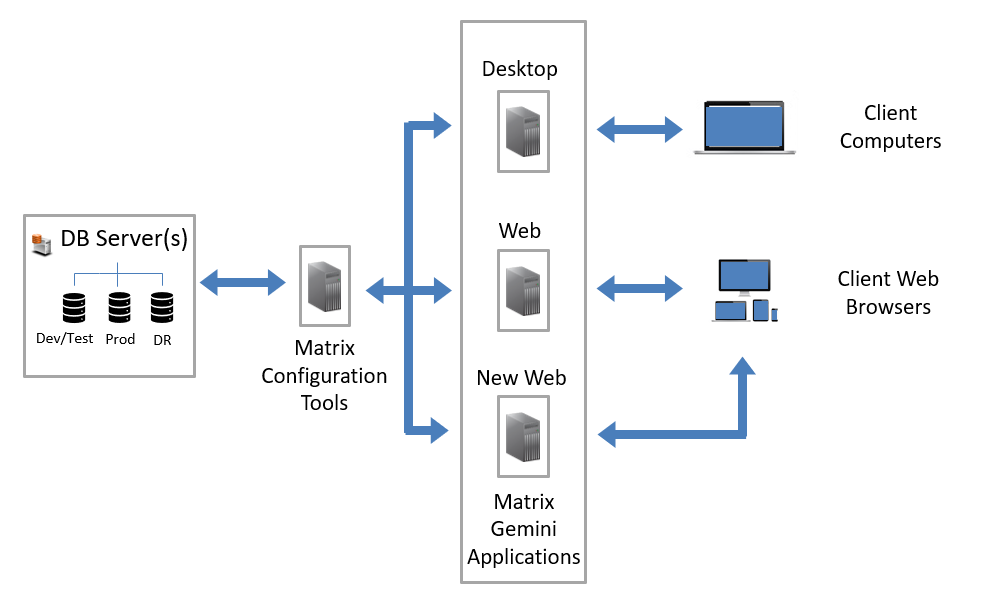 Autoscribe Informatics, the global leader in configurable Laboratory Information Management Systems (LIMS), today announced the release of Matrix Gemini version 6.5. In a major upgrade to the underlying technology Autoscribe has introduced a new web browser application for Matrix Gemini known simply as the New Matrix Gemini Web application.[Read More]
Autoscribe Informatics, the global leader in configurable Laboratory Information Management Systems (LIMS), today announced the release of Matrix Gemini version 6.5. In a major upgrade to the underlying technology Autoscribe has introduced a new web browser application for Matrix Gemini known simply as the New Matrix Gemini Web application.[Read More]
August 10, 2022 - Celebrating The Go-Live of the STARLIMS Quality Manufacturing (QM) Solution At Jiuzhou Pharma
 The project went live successfully on June 15, with an implementation cycle of only 4 months. During the severe epidemic lockdown in China, the STARLIMS team collaborated closely with the customer, coordinating resources efficiently between different parties. Under the strict quality management and project management, the STARLIMS system has been successfully delivered on time to support the customer’s long-term development. [Read More]
The project went live successfully on June 15, with an implementation cycle of only 4 months. During the severe epidemic lockdown in China, the STARLIMS team collaborated closely with the customer, coordinating resources efficiently between different parties. Under the strict quality management and project management, the STARLIMS system has been successfully delivered on time to support the customer’s long-term development. [Read More]
July 27, 2022 - Specialty Lubricant Manufacturer Benz Oil Adopts Matrix Gemini LIMS to Manage Testing and Reporting
 A new case study from Autoscribe Informatics highlights how Benz Oil adopted Matrix Gemini LIMS in its laboratory to test batches of product for quality control purposes., In addition, customers can submit field samples for routine and investigative testing. Using the new LIMS reduces the total time taken to register incoming samples from both production and external clients by up to 80%. The task that used to take 5 – 6 hours per day has been reduced to around 1 hour. [Read More]
A new case study from Autoscribe Informatics highlights how Benz Oil adopted Matrix Gemini LIMS in its laboratory to test batches of product for quality control purposes., In addition, customers can submit field samples for routine and investigative testing. Using the new LIMS reduces the total time taken to register incoming samples from both production and external clients by up to 80%. The task that used to take 5 – 6 hours per day has been reduced to around 1 hour. [Read More]