| Align Your LIMS and Org Chart to Optimize Lab Performance  When deploying your LIMS, the LabLynx team helps align your solution with your lab’s organizational structure. We work with you to identify the user profiles, management tools, and approval processes that your lab needs to run more efficiently. Once implemented, your LIMS administrators can easily reconfigure these capabilities to reflect new personnel or reporting structures. [Read More] When deploying your LIMS, the LabLynx team helps align your solution with your lab’s organizational structure. We work with you to identify the user profiles, management tools, and approval processes that your lab needs to run more efficiently. Once implemented, your LIMS administrators can easily reconfigure these capabilities to reflect new personnel or reporting structures. [Read More]
Modern Analytics Applied to Public Health  In today’s world, analytics is more important than ever and an essential part of public health. With the ongoing COVID-19 pandemic, public and health officials need to stay current, monitor trends, and make informed decisions with every tool available, especially those using data. In this article, we present concrete examples and discuss how two seasoned analytics techniques—forecasting and heat mapping—can be reapplied in new ways that weren’t available during past pandemics. [Read More] In today’s world, analytics is more important than ever and an essential part of public health. With the ongoing COVID-19 pandemic, public and health officials need to stay current, monitor trends, and make informed decisions with every tool available, especially those using data. In this article, we present concrete examples and discuss how two seasoned analytics techniques—forecasting and heat mapping—can be reapplied in new ways that weren’t available during past pandemics. [Read More]
Cloud-based LIMS - Questions you Need to Ask Prospective Providers  There is such a variety of information available about cloud-based LIMS (Laboratory Information Management System) solutions that it is time we tried to fill in the gaps and move away from the marketing hype. We hope this article will clarify some possible misconceptions, open a few eyes, and arm you with the questions you and your IT team should be asking about cloud-based LIMS. [Read More] There is such a variety of information available about cloud-based LIMS (Laboratory Information Management System) solutions that it is time we tried to fill in the gaps and move away from the marketing hype. We hope this article will clarify some possible misconceptions, open a few eyes, and arm you with the questions you and your IT team should be asking about cloud-based LIMS. [Read More]
White Paper – 6 Key Considerations When Selecting a Safety System  We are at an interesting time in today’s Pharmacovigilance (PV) landscape as there are several new systems coming to market as well as significant upgrades to systems already in the market. A strong Pharmacovigilance (PV) selection process will set you up for not only a system that meets your requirements but also a successful implementation. [Read More] We are at an interesting time in today’s Pharmacovigilance (PV) landscape as there are several new systems coming to market as well as significant upgrades to systems already in the market. A strong Pharmacovigilance (PV) selection process will set you up for not only a system that meets your requirements but also a successful implementation. [Read More]
QLIMS Business Intelligence – 2  Welcome to the second part of our series on QLIMS Business Intelligence. In our previous article, we focused on Dashboards, Visualisations, and the powerful Kibana. In this second part of our QLIMS series, we will delve into the platform’s SaaS solution, maintenance, and development. [Read More] Welcome to the second part of our series on QLIMS Business Intelligence. In our previous article, we focused on Dashboards, Visualisations, and the powerful Kibana. In this second part of our QLIMS series, we will delve into the platform’s SaaS solution, maintenance, and development. [Read More]
Consumer Complacency with Food Safety Alerts  We rely on food testing labs to guarantee the security of our food supply chain at all points within the food distribution system. Food-borne illness outbreaks can have disastrous consequences and implications for public health, but what notification systems exist for these scenarios? Does the consumer even consider these notifications before acting? [Read More] We rely on food testing labs to guarantee the security of our food supply chain at all points within the food distribution system. Food-borne illness outbreaks can have disastrous consequences and implications for public health, but what notification systems exist for these scenarios? Does the consumer even consider these notifications before acting? [Read More]
Astrix Podcast – Insights to Succeed Throughout the Regulatory Information Management (RIM) Journey  Ensuring timely and accurate Regulatory submissions can be a daunting task. The volume of processes, systems, and supporting tools needed to plan, track, author, publish, and submit to Health Authorities can be staggering. The process can be more complex when global affiliates require Rest of World (ROW) submissions. [Read More] Ensuring timely and accurate Regulatory submissions can be a daunting task. The volume of processes, systems, and supporting tools needed to plan, track, author, publish, and submit to Health Authorities can be staggering. The process can be more complex when global affiliates require Rest of World (ROW) submissions. [Read More]
LIMS for Research Laboratories  Managing the day-to-day operations of a research laboratory is a very challenging task. It requires speed, efficiency, and flexibility to keep up in a fast-paced and ever-changing environment. This being why an advanced Laboratory Information Management System (LIMS) is essential in helping research labs streamline operations while maintaining the highest standards of data integrity. [Read More] Managing the day-to-day operations of a research laboratory is a very challenging task. It requires speed, efficiency, and flexibility to keep up in a fast-paced and ever-changing environment. This being why an advanced Laboratory Information Management System (LIMS) is essential in helping research labs streamline operations while maintaining the highest standards of data integrity. [Read More]
Autoscribe Expands into Egypt Appointing Labtec Egypt Group as a Distributor 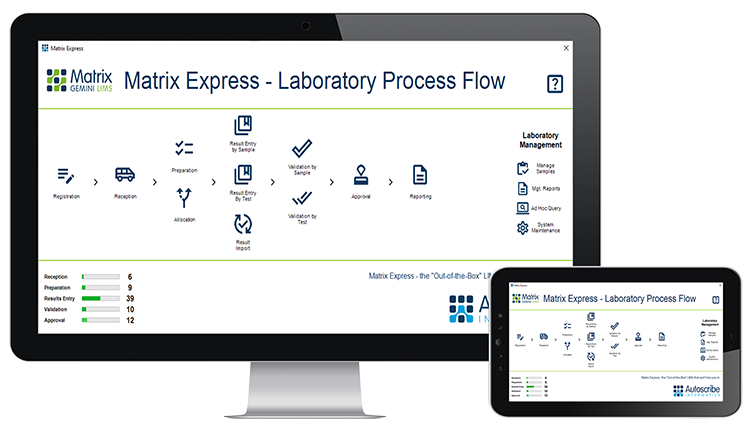 Autoscribe Informatics has appointed Labtec Egypt Group (LEG) as its distributor for the Matrix Gemini Laboratory Information Management System (LIMS) in Egypt. Founded in 1993 by Dr.Ehab Adeeb, LEG is recognized today as one of the leading professional companies in Egypt, known for deep technical knowledge, high quality support, and specialization in most laboratory supplies and services. [Read More] Autoscribe Informatics has appointed Labtec Egypt Group (LEG) as its distributor for the Matrix Gemini Laboratory Information Management System (LIMS) in Egypt. Founded in 1993 by Dr.Ehab Adeeb, LEG is recognized today as one of the leading professional companies in Egypt, known for deep technical knowledge, high quality support, and specialization in most laboratory supplies and services. [Read More]
|