| 01/11/2023 - Laboratories Must Start Exploiting the Benefits of Digitalization  As a tight labor market makes it difficult to find staff, and tougher economic conditions start to bite, laboratory managers are being forced to think hard about driving efficiency. Using the power of computer-based laboratory informatics solutions provides an easy win, but many organizations are still not fully exploiting the benefits that solutions such as LIMS offer. Reducing the number of manual ‘touch points’ and interventions for samples and their data is key to driving up efficiency. [Read More] As a tight labor market makes it difficult to find staff, and tougher economic conditions start to bite, laboratory managers are being forced to think hard about driving efficiency. Using the power of computer-based laboratory informatics solutions provides an easy win, but many organizations are still not fully exploiting the benefits that solutions such as LIMS offer. Reducing the number of manual ‘touch points’ and interventions for samples and their data is key to driving up efficiency. [Read More]
01/11/2023 - The Return on Investment for Laboratory Informatics  Laboratory work depends upon highly skilled people and sophisticated equipment to produce knowledge, information, and data. The cost, value, and ROI for those products are affected by the methods used to record and manage them. Producing knowledge, information, and data is one key aspect of laboratory work. Keeping it useable, accessible, and searchable over the long term is key to maintaining its value and realizing the return on your investment in laboratory space, equipment, and personnel. [Read More] Laboratory work depends upon highly skilled people and sophisticated equipment to produce knowledge, information, and data. The cost, value, and ROI for those products are affected by the methods used to record and manage them. Producing knowledge, information, and data is one key aspect of laboratory work. Keeping it useable, accessible, and searchable over the long term is key to maintaining its value and realizing the return on your investment in laboratory space, equipment, and personnel. [Read More]
01/11/2023 - How Logilab SDMS helps Laboratories to enable Eudralex Annex 11 Compliance: Part 3  This annex applies to all forms of computerised systems used as part of a GMP regulated activities. A computerised system is a set of software and hardware components which together fulfill certain functionalities. The application should be validated; IT infrastructure should be qualified. Where a computerised system replaces a manual operation, there should be no resultant decrease in product quality, process control or quality assurance. There should be no increase in the overall risk of the process. [Read More] This annex applies to all forms of computerised systems used as part of a GMP regulated activities. A computerised system is a set of software and hardware components which together fulfill certain functionalities. The application should be validated; IT infrastructure should be qualified. Where a computerised system replaces a manual operation, there should be no resultant decrease in product quality, process control or quality assurance. There should be no increase in the overall risk of the process. [Read More]
01/11/2023 - Getting the Case Intake Process Right in Your Safety Systems  The Intake process and its corresponding tools have been a point of focus for companies updating their safety systems because the Case Intake (Initial Triage) Workflow phase can take up to 50% of the overall case processing time. While some companies are taking a “Big Bang” approach to evaluating (and replacing) safety systems, many look to maximize their investments by limiting their focus to improving or replacing their Intake tool. [Read More] The Intake process and its corresponding tools have been a point of focus for companies updating their safety systems because the Case Intake (Initial Triage) Workflow phase can take up to 50% of the overall case processing time. While some companies are taking a “Big Bang” approach to evaluating (and replacing) safety systems, many look to maximize their investments by limiting their focus to improving or replacing their Intake tool. [Read More]
01/11/2023 - Think a LIMS Is Not for Your Industry? - Think Again 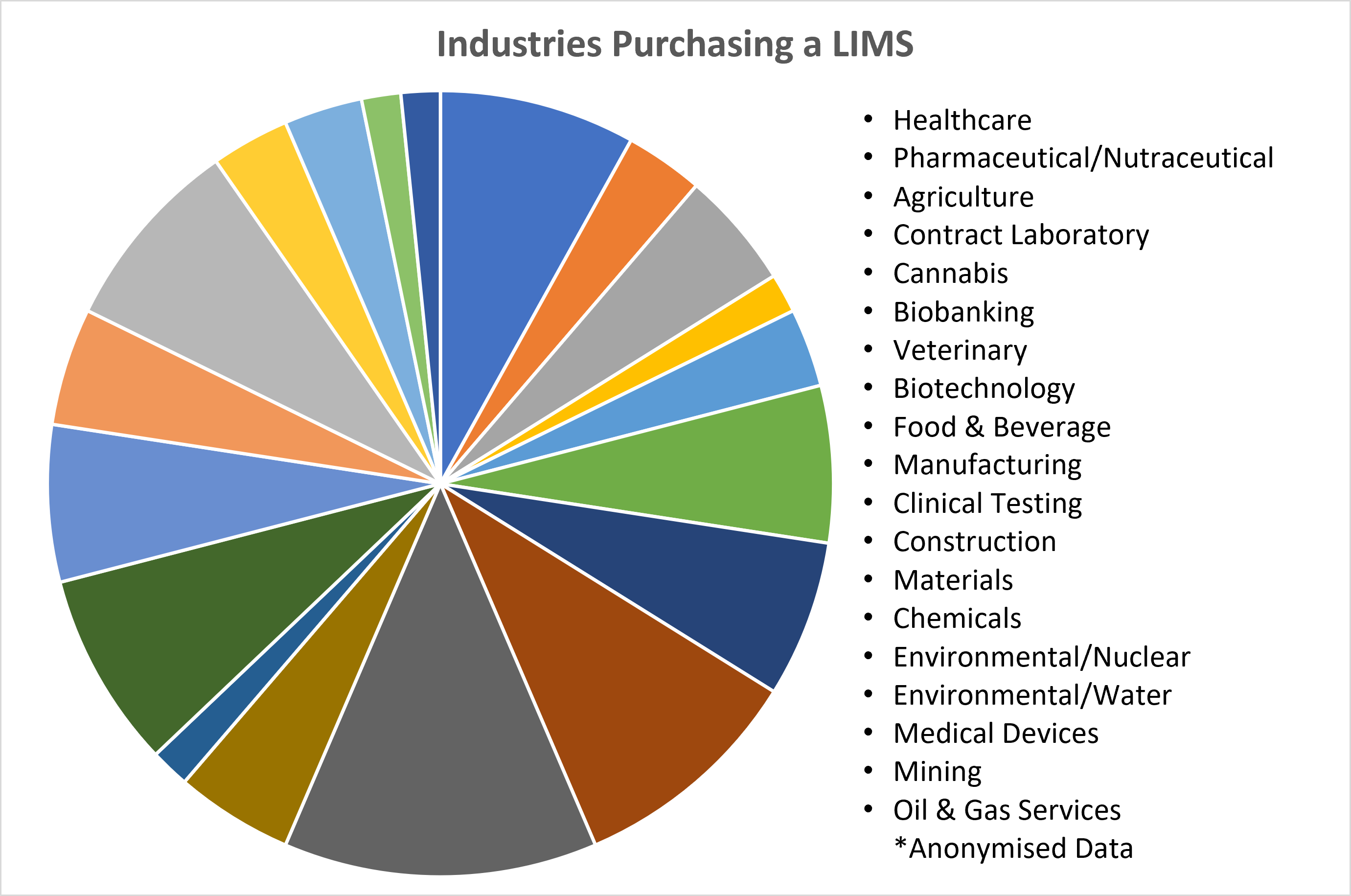 Recent data released by Autoscribe Informatics shows the breadth of industries purchasing a Laboratory Information Management System (LIMS); many for the first time. This market analysis from recent projects shows the cross-section of clients is moving well beyond what many think of as the LIMS core market of Healthcare, Medical Devices, and Pharmaceuticals. [Read More] Recent data released by Autoscribe Informatics shows the breadth of industries purchasing a Laboratory Information Management System (LIMS); many for the first time. This market analysis from recent projects shows the cross-section of clients is moving well beyond what many think of as the LIMS core market of Healthcare, Medical Devices, and Pharmaceuticals. [Read More]
|