| 02/23/2022 - SCC Soft Computer’s Blood Bank Product Receives 510(k) Clearance from the U.S. Food and Drug Administration  SCC Soft Computer’s SoftDonor.web™ version 4.5.5.0 received 510(k) clearance from the U.S. Food and Drug Administration (FDA). SoftDonor.web™ has enhanced features to increase productivity in the laboratory while maintaining compliance with safety regulations. [Read More] SCC Soft Computer’s SoftDonor.web™ version 4.5.5.0 received 510(k) clearance from the U.S. Food and Drug Administration (FDA). SoftDonor.web™ has enhanced features to increase productivity in the laboratory while maintaining compliance with safety regulations. [Read More]
02/23/2022 - Artificial Intelligence and Machine Learning: A New Frontier  As the everyday world becomes immersed in technology and we become increasingly dependent on computers to assist in day-to-day tasks, the field of life sciences is following the same path of utilizing artificial intelligence (AI). [Read More] As the everyday world becomes immersed in technology and we become increasingly dependent on computers to assist in day-to-day tasks, the field of life sciences is following the same path of utilizing artificial intelligence (AI). [Read More]
02/23/2022 - Testing the Limits in LIMS - Part 1 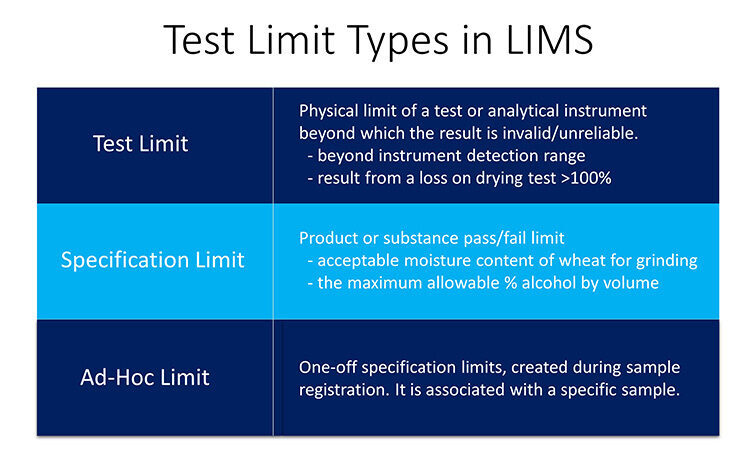 A two-part series covering what limits are and how to apply them in a LIMS. One of the many benefits of implementing a Laboratory Information Management System (LIMS) is the reduction, or elimination, of potential user error and the consistent application of standard operating procedures. [Read More] A two-part series covering what limits are and how to apply them in a LIMS. One of the many benefits of implementing a Laboratory Information Management System (LIMS) is the reduction, or elimination, of potential user error and the consistent application of standard operating procedures. [Read More]
02/23/2022 - What Labs Need to Know About Risk Management in Software  Every time we ride a bicycle, drive a car, or travel by plane, we’re faced with the risk of injury. While we can’t completely eliminate all the risks, we can reduce our chances of getting hurt — think bicycle helmets, seat belts, and oxygen masks. Similarly, clinical diagnostic labs have to accept a certain amount of risk (known as risk acceptance), but there are a number of ways these risks can be mitigated. Every time we ride a bicycle, drive a car, or travel by plane, we’re faced with the risk of injury. While we can’t completely eliminate all the risks, we can reduce our chances of getting hurt — think bicycle helmets, seat belts, and oxygen masks. Similarly, clinical diagnostic labs have to accept a certain amount of risk (known as risk acceptance), but there are a number of ways these risks can be mitigated.
[Read More]
02/23/2022 - 5 Key Benefits of a Unified LIS for All Medical Lab Disciplines  Outstanding healthcare begins with effective management of patient data from multiple sources across a network of medical care providers and organizations. Enabling health care providers to enter and access patient information via a unified, secure database provides a holistic view to better inform medical decisions and the quality of care. Outstanding healthcare begins with effective management of patient data from multiple sources across a network of medical care providers and organizations. Enabling health care providers to enter and access patient information via a unified, secure database provides a holistic view to better inform medical decisions and the quality of care.
[Read More]
02/23/2022 - FDA Food Safety Modernization Act Final Rule on Laboratory Accreditation for Analyses of Foods: Considerations for Labs and Informatics Vendors .jpg) On December 3, 2021, the the U.S. Food and Drug Administration (FDA) published it's final rule concerning the Laboratory Accreditation for Analyses of Foods (LAAF) program. Effective February 1, 2022, this rule enables the accreditation of interested food and environmental testing laboratories for specific types of testing of the U.S. food supply. [Read More] On December 3, 2021, the the U.S. Food and Drug Administration (FDA) published it's final rule concerning the Laboratory Accreditation for Analyses of Foods (LAAF) program. Effective February 1, 2022, this rule enables the accreditation of interested food and environmental testing laboratories for specific types of testing of the U.S. food supply. [Read More]
02/23/2022 - Agilent Acquires Artificial Intelligence Technology to Enhance Lab Productivity  Agilent Technologies Inc. today announced it has acquired advanced artificial intelligence (AI) technology developed by Virtual Control, an AI and machine learning software developer that creates innovative analysis solutions in lab testing. [Read More] Agilent Technologies Inc. today announced it has acquired advanced artificial intelligence (AI) technology developed by Virtual Control, an AI and machine learning software developer that creates innovative analysis solutions in lab testing. [Read More]
02/09/2022 - Build a Tomorrow-Ready Lab with LIMS  Building a digital strategy that addresses the needs & trends of tomorrow is crucial to effective digital transformation. Quality, Compliance, and Automation are the three key themes that will influence today’s digital decisions. Read this whitepaper to learn more. [Read More] Building a digital strategy that addresses the needs & trends of tomorrow is crucial to effective digital transformation. Quality, Compliance, and Automation are the three key themes that will influence today’s digital decisions. Read this whitepaper to learn more. [Read More]
|