| 01/26/2022 - Why Data Integrity is Needed for a Digital Transformation  To be effective, digital transformation efforts to improve business operations require reliable data. The exponential growth of business data, along with advances in cloud computing, artificial intelligence, and the Internet of Things, has ushered in a new era of digital transformation around the world – making... [Read More] To be effective, digital transformation efforts to improve business operations require reliable data. The exponential growth of business data, along with advances in cloud computing, artificial intelligence, and the Internet of Things, has ushered in a new era of digital transformation around the world – making... [Read More]
01/26/2022 - Software Quality: What Role Does Testing Play?  No discussion about software quality would be complete without examining the role of software testing. Let's take a closer look at which types of laboratory software need to be tested, when they should be tested, and how testing can help you ensure the quality of your software is high. No discussion about software quality would be complete without examining the role of software testing. Let's take a closer look at which types of laboratory software need to be tested, when they should be tested, and how testing can help you ensure the quality of your software is high.
[Read More]
01/26/2022 - Manufacturing LIMS Webinar Draws a Crowd 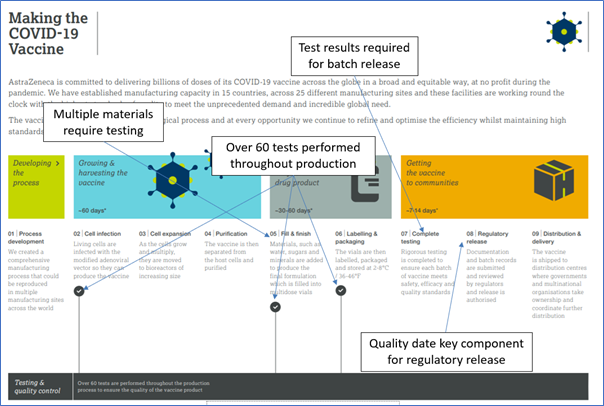 One of the best attended Autoscribe Informatics’ educational webinars in 2021 was the webinar on LIMS for manufacturing environments, titled: ‘Unraveling the role of LIMS in a Manufacturing Environment’. [Read More] One of the best attended Autoscribe Informatics’ educational webinars in 2021 was the webinar on LIMS for manufacturing environments, titled: ‘Unraveling the role of LIMS in a Manufacturing Environment’. [Read More]
01/26/2022 - Stira Pharmaceuticals Selects Xybion Digital to Transform Lab Operations  Xybion Digital, a global, low-code SaaS company that enables digital transformation in highly regulated industries like Life Sciences, announced today it has been selected by Stira Pharmaceuticals to digitize their R&D and Manufacturing labs to enhance process efficiency and improve compliance. [Read More] Xybion Digital, a global, low-code SaaS company that enables digital transformation in highly regulated industries like Life Sciences, announced today it has been selected by Stira Pharmaceuticals to digitize their R&D and Manufacturing labs to enhance process efficiency and improve compliance. [Read More]
|