| 08/31/2022 - SampleVision™ v3.0—Access to Lab Results from Anywhere for FDA Regulated Environments  LIMS Wizards, LLC, a global scientific software solutions provider, announces the launch of version 3.0 of SampleVision. The latest version offers full regulatory compliance for life sciences customers via audit trail and electronic signatures as well as cutting-edge features like geolocation services, dashboards, configurable reports, image capture and barcoding. [Read More] LIMS Wizards, LLC, a global scientific software solutions provider, announces the launch of version 3.0 of SampleVision. The latest version offers full regulatory compliance for life sciences customers via audit trail and electronic signatures as well as cutting-edge features like geolocation services, dashboards, configurable reports, image capture and barcoding. [Read More]
08/31/2022 - LIMS in Manufacturing  The history of laboratory informatics began in earnest with the commercialization of instrument data systems and LIMS software, the latter having the larger impact since it could affect the entire lab’s productivity and benefit the organization it supports. That software found a home in both industrial Research & Development, Manufacturing, and Healthcare (clinical laboratories). In each of these situations, LIMS provided a means of organizing and managing testing workflows and streamlining operations. [Read More] The history of laboratory informatics began in earnest with the commercialization of instrument data systems and LIMS software, the latter having the larger impact since it could affect the entire lab’s productivity and benefit the organization it supports. That software found a home in both industrial Research & Development, Manufacturing, and Healthcare (clinical laboratories). In each of these situations, LIMS provided a means of organizing and managing testing workflows and streamlining operations. [Read More]
08/31/2022 - Using LIMS Chain of Custody to Track Items and Ownership 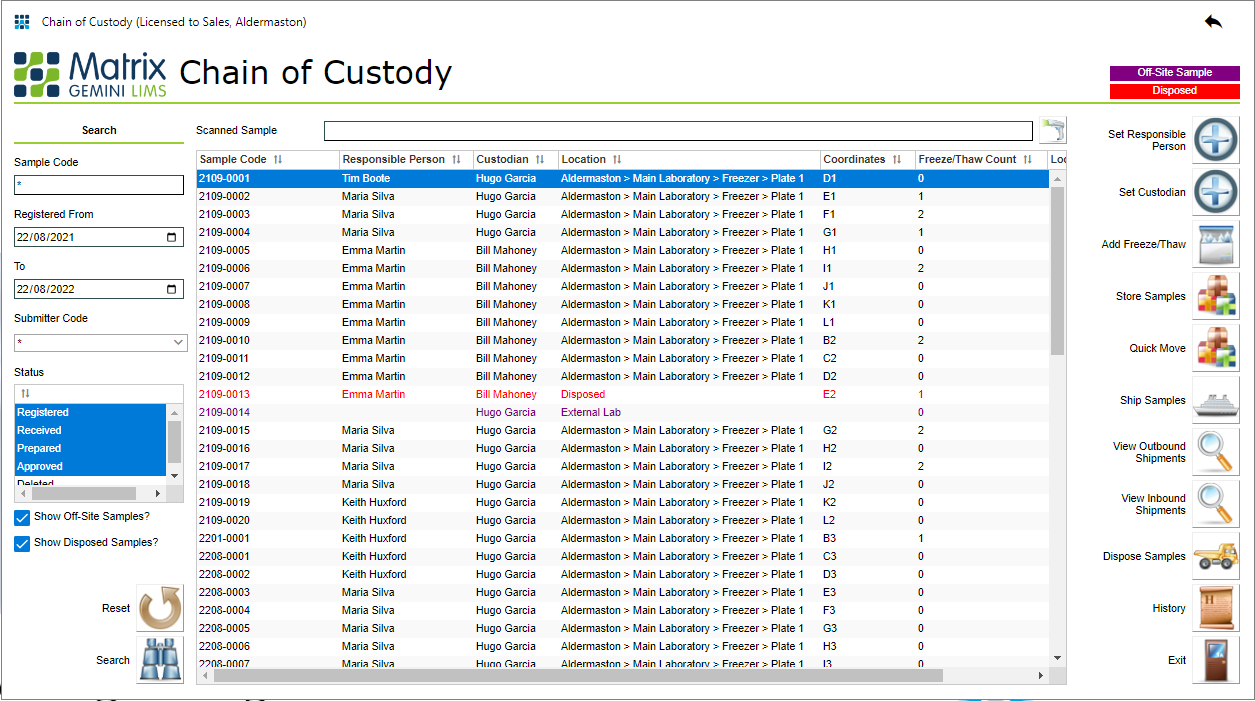 Automatically recording the Chain of Custody (CoC) for an item is essential when it is necessary to track an item throughout its lifetime. A CoC will, for example record the location of an item, showing where it is now as well as where it has been. A comprehensive CoC, however, must record more than just the location history of an item; for example, it can be important to know who is, and who has been, responsible for an item during its lifetime. [Read More] Automatically recording the Chain of Custody (CoC) for an item is essential when it is necessary to track an item throughout its lifetime. A CoC will, for example record the location of an item, showing where it is now as well as where it has been. A comprehensive CoC, however, must record more than just the location history of an item; for example, it can be important to know who is, and who has been, responsible for an item during its lifetime. [Read More]
08/31/2022 - Thermo Fisher Scientific Launches CE-IVD (IVDD) Next-Generation Sequencing Test and Analysis Software  Next-generation sequencing (NGS) is quickly becoming the platform of choice for tumor molecular profiling due to its ability to simultaneously report on multiple biomarkers. However, lengthy turnaround times can limit the clinical utility of these results. To meet the need for rapid genomic insights, Thermo Fisher Scientific today launched the CE-IVD (IVDD) Oncomine Dx Express Test and Oncomine Reporter Dx for use in clinical labs. [Read More] Next-generation sequencing (NGS) is quickly becoming the platform of choice for tumor molecular profiling due to its ability to simultaneously report on multiple biomarkers. However, lengthy turnaround times can limit the clinical utility of these results. To meet the need for rapid genomic insights, Thermo Fisher Scientific today launched the CE-IVD (IVDD) Oncomine Dx Express Test and Oncomine Reporter Dx for use in clinical labs. [Read More]
08/31/2022 - How Logilab ELN helps organizations to follow FAIR Principles  In the modern industrial era, data sharing provides a great stimulus to scientific advancement by enhancing transparency, improving collaboration, accelerating research and driving better decision-making. In areas such as public health, data sharing is very vital and critical during emergency situations such as outbreaks of infectious diseases. [Read More] In the modern industrial era, data sharing provides a great stimulus to scientific advancement by enhancing transparency, improving collaboration, accelerating research and driving better decision-making. In areas such as public health, data sharing is very vital and critical during emergency situations such as outbreaks of infectious diseases. [Read More]
08/31/2022 - The Pharma Industry Strives to Become Carbon Neutral, and LIMS Can Help  The biopharmaceutical industry can become sustainable. Several leading drug developers are determined to achieve net zero carbon emissions within the next 25 years and are enhancing efficiency in all aspects of their operations to meet this goal. In the lab, using a lab information management system (LIMS) to track and manage assets, data and reports is one way to do that. [Read More] The biopharmaceutical industry can become sustainable. Several leading drug developers are determined to achieve net zero carbon emissions within the next 25 years and are enhancing efficiency in all aspects of their operations to meet this goal. In the lab, using a lab information management system (LIMS) to track and manage assets, data and reports is one way to do that. [Read More]
08/31/2022 - How much is your hiring process costing you? Insights for business leaders  In today’s job market, where candidates have more choices than ever before, companies must focus on creating a positive and fast hiring process. How can you improve your hiring process? Below are questions that will help you identify areas where your process may need improvement. [Read More] In today’s job market, where candidates have more choices than ever before, companies must focus on creating a positive and fast hiring process. How can you improve your hiring process? Below are questions that will help you identify areas where your process may need improvement. [Read More]
|