 | | February 7, 2018
Laboratory Informatics Weekly Update | Volume 16, Issue 6 | | | | 02/22/2018 - Practical Enterprise Architecture for the Laboratory
03/01/2018 - Webinar: A Guide for Laboratory Systems Management, Part 4: LIMS/LIS, ELN, SDMS, IT & Education | | Open source data logger for low-cost environmental monitoring 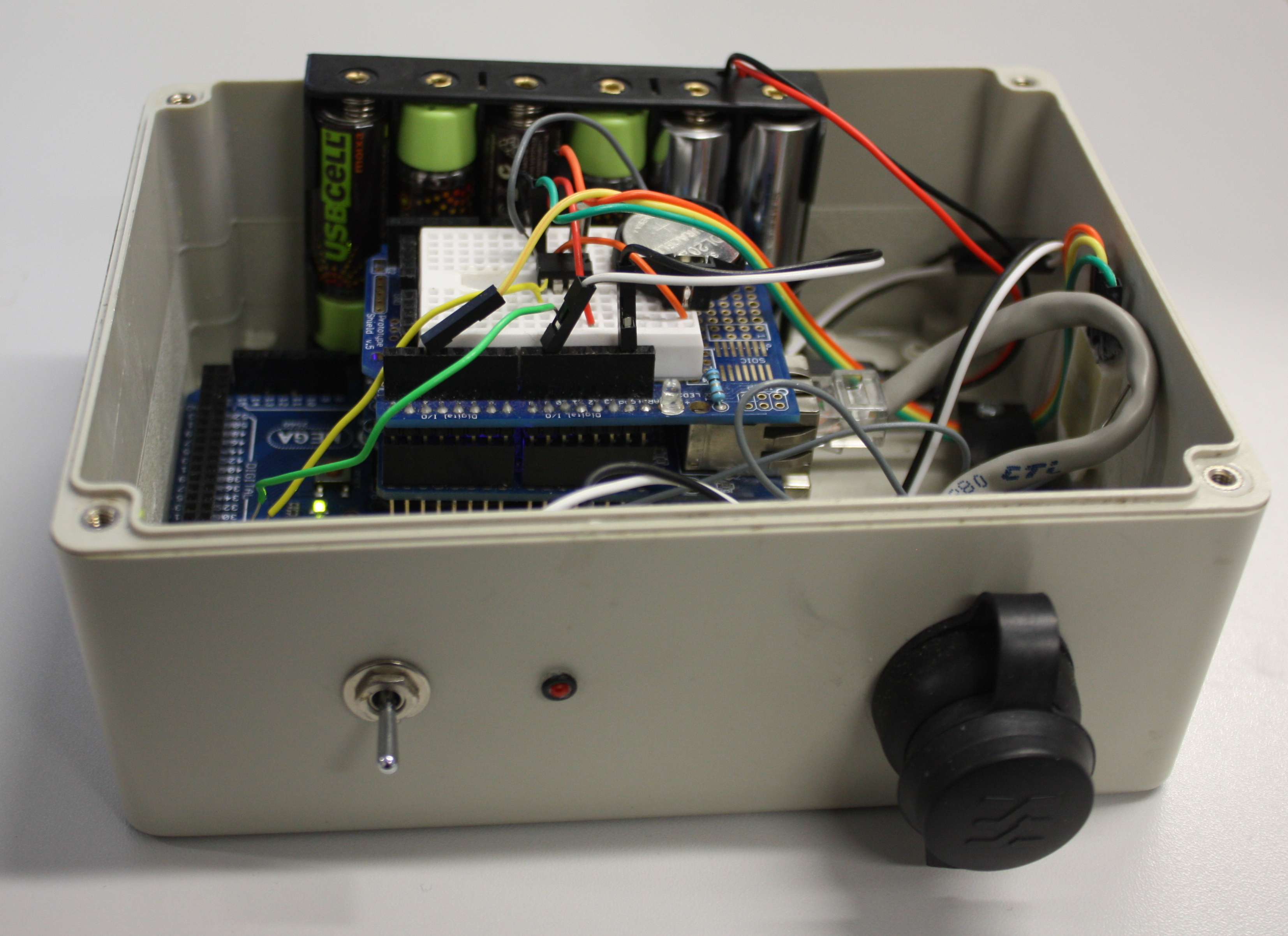 This 2014 paper by Ed Baker of London's Natural History Museum outlines a methodology for combining open-source software such as Drupal with open hardware like Arduino to create a real-time environmental monitoring station that is low-power and low-cost. Baker outlines step by step his approach (he calls it a "how to guide") to creating an open-source environmental data logger that incorporates a digital temperature and humidity sensor. Though he offers no formal conclusions, Baker states: "It is hoped that the publication of this device will encourage biodiversity scientists to collaborate outside of their discipline, whether it be with citizen engineers or professional academics." This 2014 paper by Ed Baker of London's Natural History Museum outlines a methodology for combining open-source software such as Drupal with open hardware like Arduino to create a real-time environmental monitoring station that is low-power and low-cost. Baker outlines step by step his approach (he calls it a "how to guide") to creating an open-source environmental data logger that incorporates a digital temperature and humidity sensor. Though he offers no formal conclusions, Baker states: "It is hoped that the publication of this device will encourage biodiversity scientists to collaborate outside of their discipline, whether it be with citizen engineers or professional academics."
adLIMS: A customized open source software that allows bridging clinical and basic molecular research studies 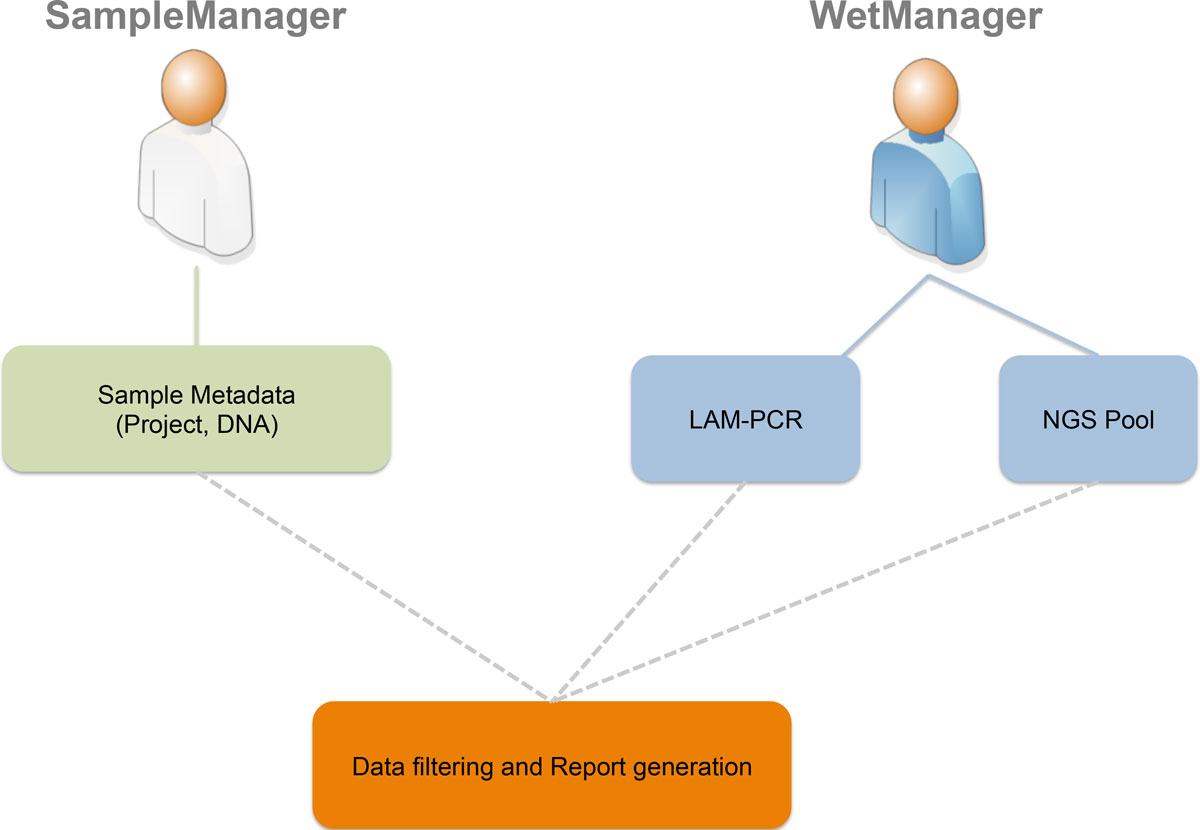 The San Raffaele Telethon Institute for Gene Therapy wanted a new LIMS to manage the data coming from their PCR techniques and next generation sequencing (NGS) methods. Not finding something suitable, the group developed its own LIMS, adLIMS. This 2015 research paper by Calabria et al., published in BMC Bioinformatics, covers the academic aspects of the information collection and development process. The San Raffaele Telethon Institute for Gene Therapy wanted a new LIMS to manage the data coming from their PCR techniques and next generation sequencing (NGS) methods. Not finding something suitable, the group developed its own LIMS, adLIMS. This 2015 research paper by Calabria et al., published in BMC Bioinformatics, covers the academic aspects of the information collection and development process. | | I Dream of IoT  | | 02/06/2018 - ISO, LIMS and Audit
01/15/2018 - About LIMS
01/02/2018 - ISO/IEC 17025-2017 Process Approach
12/14/2017 - ISO/IEC 17025:2017 – First impressions from Eurachem | | Tips for SampleManager LIMS™ Instrument Integration The following blog will detail SampleManager LIMS Instrument integration best practices and procedures to ensure success. Technological advances in laboratory instrumentation, along with higher throughput processes, have led to massive increases in the volume of analytical data. One of the main challenges faced by organizations today is turning this vast amount of data into useful information that fuels innovation and enables timely and effective business decisions. In order to manage and process these growing data volumes effectively, laboratories are looking to automate and integrate laboratory operations and processes as much as possible.
Selecting an ELN to Maximize IP Protection In the race to patent or publish, time is critical. For any researcher, securing Intellectual Property (IP) is equally critical. In order to receive a patent on an invention, inventors must be able to document the work they did to prove that the invention substantially performs against the claim. Additionally, this proof must be corroborated by someone (e.g., custodian of records) not directly involved in the work. Effective documentation and corroboration of the work done on an invention is also necessary to protect IP against challenges to the U.S. Patent and Trademark Office (USPTO) and possible litigation from competing inventors.
Ensuring the Success of Your Lab Informatics Project with the Astrix Approach™ The failure rate of Lab Informatics Projects is notoriously high. A recent survey by cloud portfolio management provider Innotas found that over half of businesses surveyed had experienced a lab informatics project failure within the previous 12 months. For any business considering a laboratory informatics project, this is not an encouraging statistic. How does one determine that a lab informatics project has failed? There are, in fact, a number of key metrics that should be used to determine success or failure.
Tips for Implementing or Upgrading Core LIMS Core Informatics provides a state of the art laboratory information management system (LIMS) built on the Platform for Science (PFS) – a Platform-as-a-Service (PaaS) solution that enables web-based informatics in the cloud. The web-based nature of Core LIMS allows for scalability, support for multiple sites, and 24-hour remote access without the need for any client-side installation. Core LIMS provides a wide array of highly configurable functionality to meet the needs of the customer. | | 02/07/2018 - Simulations Plus Launches Version 2.0 of PKPlus™
02/07/2018 - Susan Audino, Chair, Cannabis Advisory Panel and Chair, Cannabis Working Group at AOAC International, Joins the Scientific Advisory Board of CloudLIMS
02/07/2018 - Labcyte Introduces New Acoustic Sample Management Solution
02/07/2018 - Labcyte Launches New Family of Echo® Acoustic Liquid Handlers featuring 21 CFR Part 11 Compliance Software | | 02/20/2018 - SmartLab Exchange
02/26/2018 - Pittcon
03/05/2018 - HIMSS 2018
03/15/2018 - Laborama 2018
03/18/2018 - ARABLAB The Expo 2018
03/20/2018 - Paperless Lab Academy 2018 Once again, we´re setting the stage for discussions on strategies and implementation of 21th century technologies in the laboratory. We´ll discuss about the key milestones to generate solid business insights from the laboratory. Connect with colleagues who share similar operational issues as you have. Learn about user´s case studies and how they are deployed into their processes. Discover the informatics trends from cutting-edge thought leaders. Be in the know about the last informatics tools and methodologies in a creative and productive atmosphere. | | 02/14/2018 - Medical Examiner's Office Case Management Solution - Request for Proposal The purpose of this document is to solicit proposals from qualified contractors in order to obtain a third party solution that replaces the current system, addresses the needs of the Medical Examiner’s Office and best fulfills the requirements detailed in this RFP document. The services provided by the selected contractor shall include: implementation, post-implementation, maintenance and on-going support.
02/21/2018 - Solicitation - Acquisition of a LIMS for the INTS Laboratory for Medical Biology (LBM) This consultation concerns the acquisition of a LIMS for the Medical Biology Laboratory (Lbm) of the Ints. This LBM is made up of the following three entities: the department of studies of agents transmissible by the blood (Dats), the department of platelet immunology (Dip) and the specialized immuno-hematology laboratory (Ihs) of the National Reference Center for blood groups. The LIMS Acquisition includes the provision, installation, migration of existing LIMS data to the new LIMS, software configuration and user training. Maintenance services are also expected from the holder at the end of the contractual warranty period. | | Contact Us | Questions or Comments? We'd like to hear your comments and suggestions. E-mail your feedback or questions to us at cjones@limsinstitute.orgCopyright Laboratory Informatics Institute, Inc. All rights reserved.Laboratory Informatics Institute, Inc. P.O. Box 813301 Smyrna, GA 30081 770-313-8186 | |