 | | July 11, 2018
Laboratory Informatics Weekly Update | Volume 16, Issue 28 | | | | Technology transfer and true transformation: Implications for open data 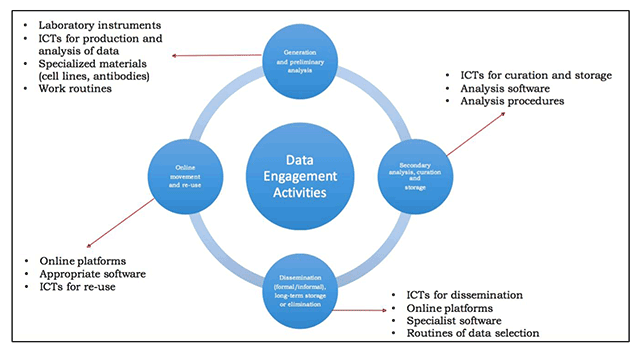 In this 2017 paper published in the Data Science Journal, University of Oxford's Louise Bezuidenhout makes a case for how local challenges with laboratory equipment, research speeds, and design principles hinder adoption of open data policies in resource-strapped countries. Noting that "openness of data online is a global priority" in research, Bezuidenhout uses interviews in various African countries and corresponding research to draw conclusions that many in high-income countries may not. The main conclusion: "Without careful and sensitive attention to [the issues stated in the paper], it is likely that [low- and middle-income country] scholars will continue to exclude themselves from opportunities to share data, thus missing out on improved visibility online." In this 2017 paper published in the Data Science Journal, University of Oxford's Louise Bezuidenhout makes a case for how local challenges with laboratory equipment, research speeds, and design principles hinder adoption of open data policies in resource-strapped countries. Noting that "openness of data online is a global priority" in research, Bezuidenhout uses interviews in various African countries and corresponding research to draw conclusions that many in high-income countries may not. The main conclusion: "Without careful and sensitive attention to [the issues stated in the paper], it is likely that [low- and middle-income country] scholars will continue to exclude themselves from opportunities to share data, thus missing out on improved visibility online."
Eleven quick tips for architecting biomedical informatics workflows with cloud computing In this educational journal article published in PLoS Computational Biology, Cole and Moore of the University of Pennsylvania's Institute for Biomedical Informatics offer 11 tips for health informatics researchers and practitioners to embrace in improving reproducibility, knowledge sharing, and costs: adopt cloud computing. The authors compare more traditional "in-house enterprise compute systems such as high-performance computing (HPC) clusters" located in academic institutions with more agile cloud computing installations, showing various ways researchers can benefit from building biomedical informatics workflows on the cloud. After sharing their tips, they conclude that "[c]loud computing offers the potential to completely transform biomedical computing by fundamentally shifting computing from local hardware and software to on-demand use of virtualized infrastructure in an environment which is accessible to all other researchers." | | A Guide for Management: Successfully Applying Laboratory Systems to Your Organization’s Work  This recorded Lab Informatics Tutorial series is designed as a management level view of laboratory systems and is appropriate for anyone planning, reviewing, or approving the acquisition of laboratory informatics. A background in science is not necessary to follow the presented material. Its purpose is to provide you with an understanding of how these technologies (Laboratory Information Management Systems, Electronic Laboratory Notebooks, Scientific Data Management Systems, Laboratory Execution Systems, Instrument Data Systems, and supporting technologies ) can be used to support/improve your labs operations, and the considerations that need to be taken into account before they are purchased. This recorded Lab Informatics Tutorial series is designed as a management level view of laboratory systems and is appropriate for anyone planning, reviewing, or approving the acquisition of laboratory informatics. A background in science is not necessary to follow the presented material. Its purpose is to provide you with an understanding of how these technologies (Laboratory Information Management Systems, Electronic Laboratory Notebooks, Scientific Data Management Systems, Laboratory Execution Systems, Instrument Data Systems, and supporting technologies ) can be used to support/improve your labs operations, and the considerations that need to be taken into account before they are purchased. | | LIMSjournal - Summer 2018: Volume 4, Issue 2  | | 07/10/2018 - California-FDA Cannabis Laboratory Method Validation Requirements for Growers, Processors, and Dispensaries
07/11/2018 - Webinar: 5 Ways Biopharma and Medical Device Manufacturers Can Accelerate Patient-Centric Innovation
07/12/2018 - Webinar: The Role of Dublin Core Metadata in the Expanding Digital and Analytical Skill Set Required by Data-Driven Organizations | | Integrating Multiple LIMS into a Single System  In today’s global economy, mergers and acquisitions have become a dominant strategy to improve profitability, maintain competitive edge, and expand services and reach. This practice is common in several industries such as pharmaceutical, biotech, food and beverage, oil and gas, and others. While corporate mergers certainly can provide several benefits for the organizations involved, they can also present significant challenges, not the least of which is harmonization and optimization of the laboratory environment. This often leads to the need to the need for integrating multiple LIMS apps to support a global enterprise.
LIMS Data Migration When implementing a new LIMS, the question is not usually whether to migrate legacy system data, but how much data, in what manner, and why?
Importance of LIMS for Biobanks Any entity that stores biological samples can be called a Biobank. However, the term ‘Biobank’ is most commonly used for entities that store biological specimens on a large scale to provide them as a service for internal or external entities like researchers. Biobanks have to offer unbiased and high-quality samples using well-designed and documented procedures. This ensures a biological specimen is kept in a controlled environment and guarantees the ethical aspect of sample collection.
What the New FDA Guidance for ICH GCP E6 R2 Means for Sponsors and CROs FDA Guidance for Good Clinical Practice (GCP) is an international quality standard defined by the International Council for Harmonization (ICH) that governs ethical and scientific considerations for designing, conducting, recording and reporting trials involving human subjects. Compliance with FDA Guidance for GCP in clinical trials helps to assure that the rights, well-being and safety of trial participants are protected and that the data generated in the trial is credible. | | 07/09/2018 - Thermo Fisher Scientific Signs Agreement to Acquire Gatan from Roper Technologies
07/09/2018 - Thermo Fisher Scientific to Provide Antibody Publication Data on its Website
07/09/2018 - Azalea Health Announces Partnership with Iron Bridge for All Public Health Submissions | | 07/29/2018 - Clinical Lab Expo 2018
09/06/2018 - India Lab Expo
09/10/2018 - Smartlab Forum
09/12/2018 - Thailand Lab International 2018
09/17/2018 - Genome Informatics 2018
10/31/2018 - Lab Innovations 2018
11/13/2018 - LIMS-Forum 2018 | | 07/11/2018 - Request for Information: Laboratory Inventory System
07/13/2018 - Expression of Interest: Framework for Laboratory Information Management Systems (LIMS)
07/18/2018 - Request for Tender: Purchase of Laboratory Information Management System (LIMS)
07/18/2018 - Invitation to Bid: Water & Wastewater Laboratory Analysis Services
07/19/2018 - Request for Quotation: Laboratory Information Management System (LIMS) Software
07/24/2018 - Invitation to Bid: Laboratory and Sampling Services for Water and Wastewater Treatment Processes
07/26/2018 - RFP/Bid - Laboratory Information Management System Unified Government of Wyandotte County and Kansas City, KS R28270 - Laboratory Information Management System
07/26/2018 - Contract Notice: NHS Lanarkshire Laboratory Management System
07/30/2018 - Request for Tender: Integrated Electronic Health and Care Record for Health and Social Care Northern Ireland | | | E-mail your feedback or questions to us at: limsforum@lablynx.com Mail us: LabLynx, Inc. P.O. Box 673966 Marietta, GA 30006 Telephone: 770-859-1992 | |