Introductory evidence on data management and practice systems of forensic autopsies in sudden and unnatural deaths: A scoping review
| Full article title | Introductory evidence on data management and practice systems of forensic autopsies in sudden and unnatural deaths: A scoping review |
|---|---|
| Journal | Egyptian Journal of Forensic Sciences |
| Author(s) | Prahladh, Salona; van Wyk, Jacqueline |
| Author affiliation(s) | Inkosi Albert Luthuli Central Hospital, University of KwaZulu-Natal |
| Primary contact | Email: prahladhs at ukzn dot ac dot za |
| Year published | 2022 |
| Volume and issue | 12 |
| Article # | 38 |
| DOI | 10.1186/s41935-022-00293-3 |
| ISSN | 2090-5939 |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://ejfs.springeropen.com/articles/10.1186/s41935-022-00293-3 |
| Download | https://ejfs.springeropen.com/counter/pdf/10.1186/s41935-022-00293-3.pdf (PDF) |
Abstract
Background: The investigation into sudden unexpected and unnatural deaths supports criminal justice, aids in litigation, and provides important information for public health, including surveillance, epidemiology, and prevention programs. The use of mortality data to convey trends can inform policy development and resource allocations. Hence, data practices and data management systems in forensic medicine are critical. This study scoped literature and described the body of knowledge on data management and practice systems in forensic medicine.
Methods: Five steps of the methodological framework of Arksey and O’Malley guided this scoping review. A combination of keywords, Boolean terms, and medical subject headings was used to search PubMed, EBSCOhost (CINAHL with full text and Health Sources), Cochrane Library, Scopus, Web of Science, Science Direct, WorldCat, and Google Scholar for peer review papers in English from June 18–24 of 2020, with an updated search also occurring in November 2021. This study included articles involving unnatural deaths, focused on data practice or data management systems, relating to forensic medicine, all study designs, and published in English. Screening, selection, and data extraction were conducted by two reviewers. Thematic analysis was conducted, and the results were reported using both quantitative and qualitative methods.
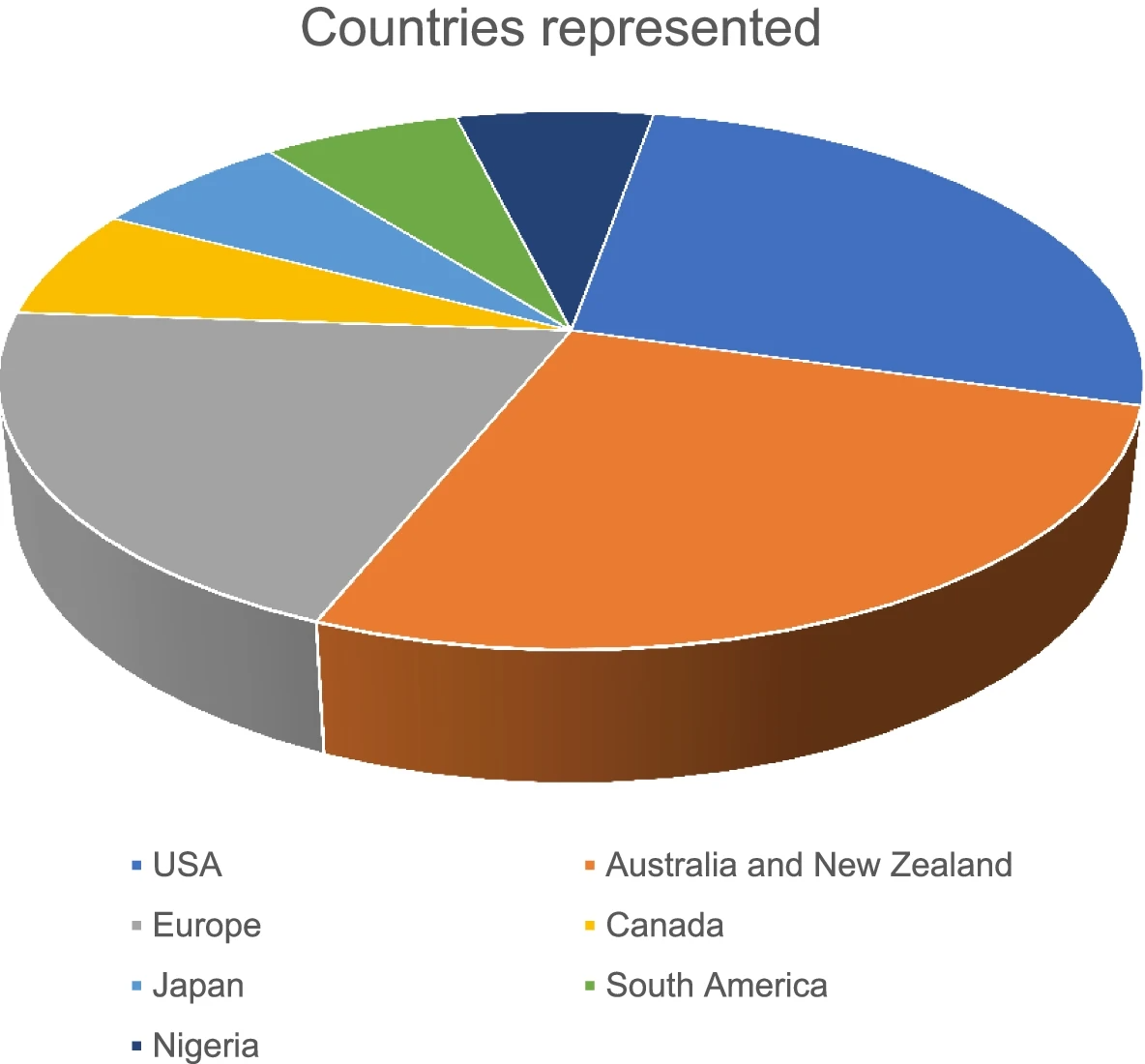
Results: Of the examined 23,059 articles, 16 met this study’s inclusion criteria. The included articles were published between 2008 and 2019. Eight of the 16 articles were published between 2017 and 2019. Most of the included studies were conducted in the United States (5) and Australia/New Zealand (4). Only two publications were from lower- and middle-income countries (LMICs; Nigeria and Mexico), and the remaining 14 were from high-income countries (Italy, Denmark, US, Australia, New Zealand, Japan, Switzerland, and Canada). The data management systems found in this study were as follows: Virtopsy, Canadian Coroner and Medical Examiner Database, Infant Injury Database, Ibadan pilot fatal injury surveillance system, Medical Examiners and Coroners Alert System, National Violent Deaths Reporting System, AM/PM Database, Tokyo CDISC/ODM, and National Coronial Information System.
Conclusions: This study’s results revealed limited articles relating to data management and practice systems in forensic medicine—particularly in LMICs—indicating there is a prevalence of unnatural deaths in LMICs. This study, therefore, recommends research on data management and practice systems relating to forensic medicine in LMICs to inform policy decisions.
Keywords: unnatural death, data practice, data management systems, autopsy, post-mortem examination, forensic medicine, pathology
Background
The global burden of trauma, particularly in low- and middle-income countries (LMICs) places a large strain on resources, and therefore, the diagnostic value of autopsies must be reiterated.[1] The use of autopsies remains the gold standard in assessing standards of medical care. There is a concerning decline in autopsies even though their value to the medical fraternity is acknowledged.[2][3] Forensic medicine and forensic pathology apply scientific and medical knowledge to inquests, and the autopsy is frequently regarded as the focus of the death investigation. The investigation into sudden unexpected and unnatural deaths supports criminal justice, aids in litigation, and provides important information for public health, including surveillance, epidemiology, and prevention programs.[3][4][5][6][7] The evidence serves to inform policy not only for injury prevention and control, but also to prevent suicide, violence, or substance abuse.[5][6][8][9][10][11]
Globally, death investigations are conducted according to prevailing legislation, which differs from country to country. Historically, the coroner system was formalized into law by England’s King Richard I in 1194, with the first coroners being knights.[12] The coroner system from England was introduced in the 1600s by American colonists, becoming an important part of the death investigation system in what would become the United States of America. However, the role of the office was later reduced to the medicolegal examination of a body and the determination of the cause and manner of death.[12] Throughout the Middle Ages, the functions of the coroner included conducting inquests, attending to and inspecting the dead, and investigating suspicious deaths.
In the US, coroners are generally public officials with minimal to no medical training. Some coroners only serve part-time capacities, and they also often have other full-time employment. The medical examiner system was introduced due to public dissatisfaction with the coroner system, accusations of corruption, and an increased need to have highly trained personnel in the death investigation.[12] This led to the emergence of a separate discipline of forensic medicine, which began in the seventeenth century.[13] The first medical examiner system was introduced in Massachusetts in 1877.
In 1959, the medical subspecialty of forensic pathology was formally certified, and medical examiners were trained in pathology. Forensic pathology is viewed as a subspecialty of anatomical pathology in countries such as Canada and the United Kingdom. In countries such as South Africa and Australia, one may train solely in forensic pathology for a minimum of a year (usually more), with additional training in anatomical pathology. In South Africa, the medicolegal death investigation is conducted primarily in terms of the Inquests Act (Act 58 of 1959). The medicolegal autopsies are performed by medical practitioners, but due to the large annual number of unnatural deaths and the small number of qualified forensic pathologists in South Africa, a large number of these autopsies are performed by colleagues with limited formal training in performing autopsies.[14]
The fundamental essence of forensic pathologists’ work is to investigate and report the cause of death. The importance of reporting the cause of death is reiterated and forms the basis of The Global Burden of Disease study.[15] This comprehensive worldwide observational epidemiological study describes mortality and morbidity from major diseases, injuries, and risk factors to health at global, national, and regional levels. Mortality reporting systems can help to prioritize health system investments, track progress towards global development goals, and guide scientific research.[15] The Global Burden of Disease study acknowledges the need for wider adoption and improvement of these systems because continuous reporting of cause-specific mortality in many countries represents a success for global health.
Information derived from autopsies has historically been paper-documented, filed, and archived. With the current age of technology, this information can be stored and managed electronically to better ensure reporting that is current, relevant, and contributory to training and service delivery, policy implementation, and social interventions. The current COVID-19 pandemic has accentuated the importance of wireless technology and the use of the internet to transcend normal communications. Due to safety reasons, much work has to be conducted remotely in many business sectors, including the medical sector. General practitioners conducted consultations virtually to adhere to social distancing and safety measures, and telephonic communication and telemedicine became a necessity due to the pandemic. At this current point in time, we are forced to be open-minded to integrate technology into our daily work lives.
This scoping review was conducted to map the evidence on data management and practice systems, their use, benefits, and challenges in forensic medicine. The information gained on the use and availability of digital technologies and their strengths and limitations to collect autopsy data can inform models to suit similar purposes in forensic medicine in LMICs.
Methods
This study’s protocol was developed a priori and published.[16] This study used the Arksey and O’Malley framework to conduct a scoping review, which includes the following: (i) the research question was identified, (ii) relevant studies were identified, (iii) eligible studies were selected, (iv) the data was charted, and (v) the results were collated and summarized.[17][18]
Identifying the research question
The main research question was “In the last 10 years, what evidence on data management and practice systems and their benefits and challenges in forensic medicine exist globally?" This study’s population, concept, and context were sudden/unnatural deaths, data practices, and forensic medicine (autopsies or post-mortem examinations) globally, respectively. The research sub-questions were as follows:
- What evidence exists on data management and practice systems in forensic medicine?
- What are the reported benefits and challenges of the data management and practice systems used in forensic medicine?
Identifying relevant studies
A systematic international search of both gray literature and published literature was done to retrieve articles relating to data practice, use, benefits, and challenges in forensic medicine. A combination of keywords, Boolean terms, and medical subject headings was used to search PubMed, EBSCOhost (Academic Search Complete, CINAHL with full text, and Health Sources), Cochrane Library, Scopus, Web of Science, Science Direct, WorldCat, and Google Scholar for peer review papers in English from June 18–24 of 2020, with an updated search also occurring in November 2021. Study design limitations were removed. The search strategy was piloted to check the appropriateness of keywords and databases. The results were reviewed by the research team to ensure the validity of the search strategy in PubMed. A manual search was conducted of the references of the included studies, and the World Health Organization (WHO) website was also searched. Each search was adequately documented, as illustrated in Supplementary File 1. The Peer Review of Electronic Search Strategies (PRESS) statement guided this study’s electronic search strategy.[19] All citations were managed using the EndNote X9 reference manager.
Selection of articles and eligibility criteria
The principal investigator conducted the database searches and title screening using this study’s eligibility criteria. The search strategy and screening tools were piloted to calibrate operators, increase consistency, and fine-tune the methods. A second reviewer reviewed the retrieved titles to ensure completeness before the abstract screening. Subsequently, the cleaned EndNote library was shared among the review team after the removal of duplicate titles. Using an electronic screening tool developed in Google forms, two reviewers independently screened the abstracts and full texts and categorized them into “include” or “exclude” categories based on this study’s eligibility criteria. The review team met throughout the screening process and resolved the discrepancies between the two reviewers at the abstract screening stage through discussions until a consensus was reached, though there were no significant disagreements among the reviewers. It was decided that the articles would be selected on a minimum agreement of at least 50% between the two reviewers due to the complex and specialized field the review would entail. The second reviewer, however, resolved the discrepancies between the principal investigator and the third reviewer at the full-text screening phase. The PRISMA flow diagram was used to account for all the articles. The eligibility criteria used in this study are outlined below:
Inclusion criteria
- Studies that involved unnatural deaths
- Studies that focused on forensic medicine (autopsies/post-mortem)
- Articles that reported data practices such as use, benefits, and challenges
- Articles published from 2008 to 2021
- Articles published in the English language
- All study designs
Exclusion criteria
- Articles that do not involve forensic medicine, pathology, and/or autopsies
- Studies with no clear targeted population
- Studies where full-text articles could not be obtained
- Articles reporting photo capture/imaging programs
- Non-English publications
Charting the data
The data for this study were collected using a spreadsheet comprising of the following: bibliographic details, publication year, study design, study setting, data practices relating to forensic medicine, uses, benefits, challenges, and conclusion and recommendations. The form was pilot tested by two reviewers independently, and all discrepancies were resolved before its usage. Finally, two reviewers performed the data extraction using both inductive and deductive approaches. Subsequent discrepancies were resolved through discussion by the review team.
Collating, summarizing, and reporting the results
Thematic content analysis was conducted for this study. The emerging themes and subthemes relating to data practices in forensic medicine were collated, summarized, and reported narratively. However, the bibliographic details of the included studies, such as design and publication year, were reported quantitatively and presented as percentages.
Results
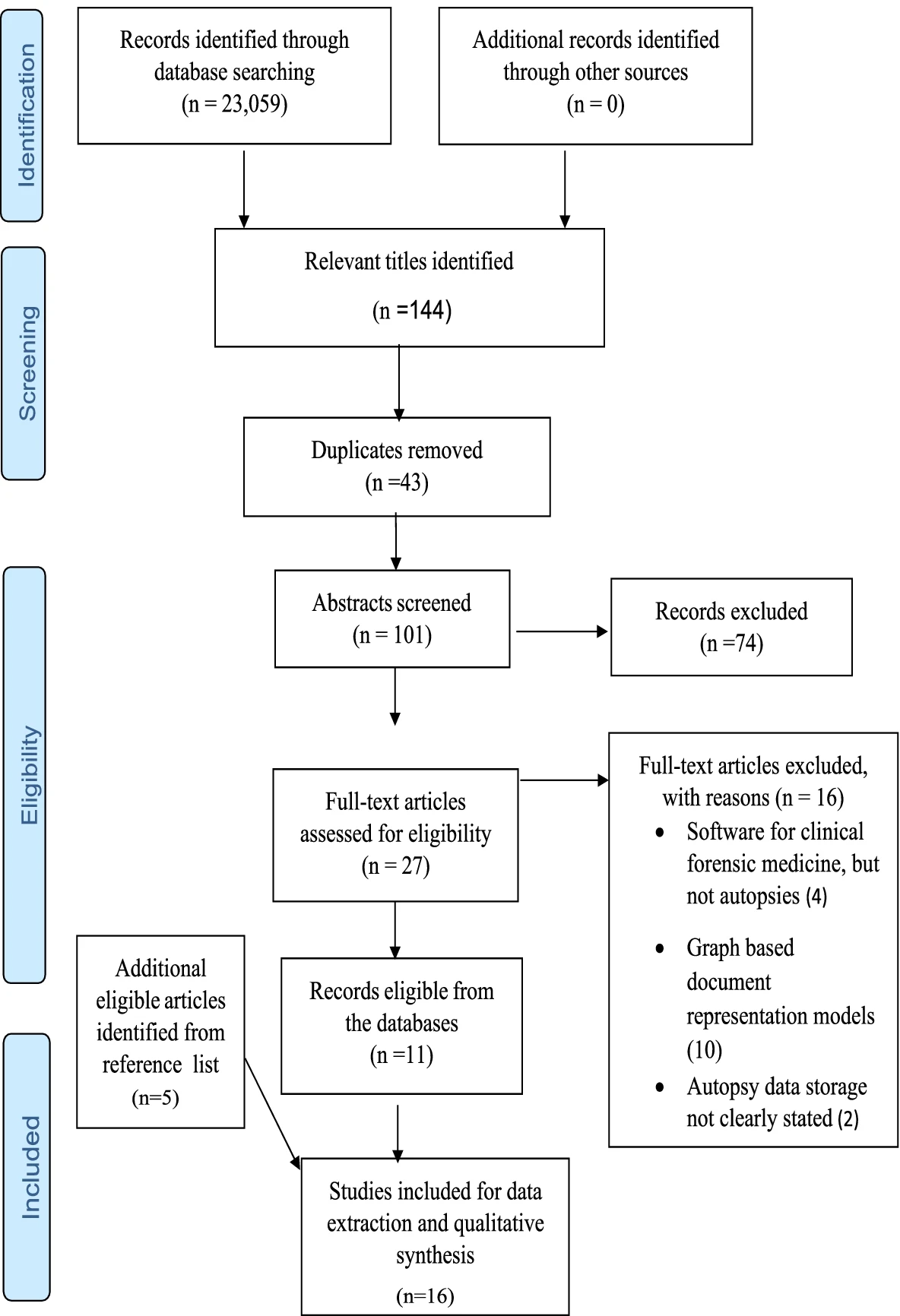
Of the total of 23,059 search yields, 144 were deemed relevant from the title screening phase based on this study’s eligibility criteria. Of the 144 titles, 43 duplicates were identified and removed, and then 74 and 16 articles were removed at the abstract and full-text screening phases, respectively. The 16 articles removed at the full-text screening phase focused on software for clinical forensic medicine, and graph-based document representation models and others did not report the data storage method. Therefore 15 articles were finally deemed eligible for inclusion for data extraction (Fig. 1). Following reviewer indication, an additional article was added to the results after the full-text screening for a total of 16 articles to be included in the study.
|
Characteristics of included publications
Table 1 provides a summary of the main characteristics of the articles included. The included articles were published between 2008 and 2019. Eight of the 16 articles were published between 2017 and 2019.[7][20][21][22][23][24][25][26] Most of the studies were published in the United States[7][21][22][27][28] and Australia/New Zealand[11][20][24][29][30], with three from European countries. Two publications were from LMICs (Nigeria and Mexico), and the remaining were from high-income countries (Italy, Denmark, United States, Australia, New Zealand, Japan, Switzerland, and Canada (Fig. 1). The article types included brief communication[26][31], original studies[20][24][25][27][32], author manuscript (preprint)[28], retrospective descriptive studies[7][21][22][23][33][34], a single systematic review[29], and an annual/government report.[35]
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Findings
Table 2 present a summary of these findings. A brief narrative of the finding is reported under the following main themes: data management and practice systems, benefits/uses of the data management and practice systems, and challenges/limitations of the data management and practice systems.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Types of data management and practice systems
All the reviewed studies described the use of electronic systems, which ranged in complexity from a simplified Excel spreadsheet[33] to a more complex system based on the use of a web portal[31], with varying use at the state and national levels, as with the National Violent Death Reporting System[28] and the National Coronial Information System.[20][24][29] The national system received full technical support. In some cases, such as mentioned in the Nigerian study, data was collected by a single user and was based on tools developed at the same time as the WHO-Monash University fatal injury surveillance manual.[33] It was found to be acceptable and timely with good data quality, which was representative and specific due to a single experienced user. Other systems sourced data from multiple users and departments such as a forensic medicine department, the police, and pychologists. Many of the systems were consolidated centralized systems that were used in forensic medicine departments (n= 10; 62.5%).[20][24][25][26][28][29][30][32][35] Access to all the systems was strictly controlled either through single- or multi-user authorization[20][25][29][31][32][34], or data are captured in a spreadsheet created in Excel.[33] The articles based on systems that reported the best utility were those that allowed greater flexibility and lend themselves to state-based use.[21][28] In these cases, the systems received government funding and technical support.[25][28][29][32][34][35]
Benefits/uses of the reported data management and practice systems
In nine (56%) of the articles, the wide coverage of cases increased the value of the system for those who used the data.[20][21][22][24][25][26][28][34][35] The benefits reported included the formulation of injury prevention policies and enhancement of epidemiological studies (n= 10; 62.5%).[20][21][22][24][26][27][28][29][34][35] Many of the cited systems contributed and supported research (n= 10; 62.5%).[17][21][24][26][27][28][29][34][35] Although only a few systems had standardization of data as an attribute (n= 4; 27%)[21][25][32][33], two reported the benefits of information security (n= 2; 13%)[31][34] and three (20%) included the anonymization of data as a characteristic.[31][32][34] Some of the systems mentioned the use of their own data quality control measures (n= 7; 47%).[20][22][24][28][29][31][35] Only six (37.5%) of the reported systems allowed for ease of data sharing/information exchange for research and policy implementation purposes.[20][24][28][29][32]
The data management and practice systems served as repositories for information to inform about international best practices and supported the development of diagnostic models.[7][21][27] The systems were also seen as a conduit for information sharing once privacy challenges were tackled.[14][27][32][34] The provision of data for research was a large contribution to most of the databases' establishment.[7][14][20][21][23][24][27][29][30][31][32][33][34] The systems contributed to the institution of preventative measures by identifying risk factors and by predicting the possible outcomes by using the data available in the reporting systems.[14][21][28][29] Improvement of surveillance and statistics related to epidemiology was notable in some studies[24][26][28], and this further informed policy changes, as well as contributed to the institution of new policies and the reformation of laws.[3][29][24][28][30]
Challenges/limitations of the data management and practice systems
In one of the systems included for review, the authors described a voluntary data capture process. This however led to inconsistencies, underreporting, and time lag that impacted the usability of the data for research and epidemiology.[18][20][21][25][33] Some of the other challenges reported (n= 8; 53%) in service work and research included underreporting, low-case numbers, or use of closed cases, only which generally impacted on epidemiology and research.[7][22][23][24][28][29][33][35] In the articles that cited data quality and validity challenges (n= 8; 53%), missing information was also reported as a limitation.[20][21][24][27][28][29][34] Data exchange and entry limitations were also reported as issues (n= 8; 53%)[20][21][24][25][27][29][32], and these barriers may in part be due to an inability to institute standardization practices for data capturing (n= 5; 33%).[21][23][25][27][28] Ethical considerations such as consent for autopsy or submitting data for analysis and research also impacted the use of the systems (n= 2; 13%).[26][31] Factors such as case-reporting bias, reporting errors, and bias in case selection also impacted case numbers and research (n= 4; 27%).[7][28][29][35]
Discussion
This study sought to map the evidence on data management and practice systems in forensic medicine. The results show that several data management and practice systems exist. However, most of the existing systems were from high-income countries, with few found in LMICs based on this study’s eligibility criteria. This review was informed by the need to collect systematic evidence on the data management and practice systems being used in forensic medicine and the possible lessons and applicability to LMIC contexts where paper reporting is still being used. The lack of information on data management and practice systems is visible in that only two of the articles included for review reported on studies from the LMIC context. Half the publications (50%) were published during and after 2017. This trend may be due to the greater access, use, and reporting of electronic data reporting systems. The inclusion of forensic pathology reports on databases also captures information that is not routinely captured in vital registration statistics. The existing data management systems in forensic medicine reported in the included articles have several benefits. Nonetheless, the included articles also reported several challenges about those existing data management systems (see Fig. 2).
|
To the best of our knowledge, this scoping review is the first present evidence on data management and practice systems in forensic medicine. Therefore, we cannot compare our findings. Nonetheless, the literature shows that electronic data reporting systems are relevant and were developed from the recognition of coronial data as not only a part of the death investigation but as a contributor to preventable death research and public health initiatives. For instance, in an article by Bruce Levy[27], which discussed the United States' systems currently in place to support forensic pathology and death investigation, the Centers for Disease Control and Prevention (CDC) implemented the National Violent Death Reporting System in response to a report describing the need for a national fatal intentional injury surveillance system. Initially, the system started in six states but later was expanded to 18 states, as stated in the publication.[21] According to the CDC website, and an article published in 2019 regarding its future directions, the program has now expanded to all 50 states and is constantly being updated and improved for data sharing.[28][36] Other systems developed in the US included a state-wide comprehensive multisource drug overdose fatality surveillance system in Kentucky (developed in response to drug overdoses cited as a public health crisis) and a database related to infant and child abuse.[7][22] The burden of sudden infant death syndrome (SIDS) and the large number of cases that remain unexplained led to the passing of legislation in Italy (2006) that fetuses and infants, from 25 weeks of gestation to one postnatal year, who died suddenly and unexpectedly should be sent to the University of Milan, Italy, for a postmortem, with parental consent.[26] An Italian research center developed a web portal for a national bank registry which has been set up to centralize records retrieved from regions across Italy, which hopes to contribute data for epidemiology and study into risk factors for SIDS and other sudden unexpected deaths in infants.
Other examples were found. For example, Canada instituted a National Coroner and Medical Examiner database to detect emerging trends and hazards for the prevention of avoidable deaths.[35] Tokyo is recognized as a technology hub, and the latest inclusion of a legal medicine information system for forensic systems is discussed in an included article.[31] Using the information system, forensic pathologists and other staff can register and search for institutional autopsy information, print death certificates, and extract data for research and analysis. Switzerland created a tool called Virtopsy, a centralized database in forensic medicine for analysis and comparison of radiological and autopsy findings.[32] It is a database currently created but not in routine use, as it has not been validated. The database compares autopsy and radiological data with photograph storage. In the Nigerian study, recognition of the poorly representative mortality injury surveillance system prompted the authors to institute an electronic injury surveillance system.[33] The system included features based on a separate South African initiative, namely the National Injury Mortality Surveillance System (NIMSS), which due to logistical reasons and lack of funding was deemed unfeasible and discontinued.[10] It should be noted that articles related to NIMSS did not meet the inclusion criteria of the current study. While the NIMSS-based tools that were utilized were already existing, making the system feasible and sustainable, an appropriate infrastructure needed to be in place to maintain the system.[10]
The recognition and understanding of violent deaths require the collection of accurate, timely, and comprehensive surveillance data to implement preventative measures.[10][14][24][28][30] The wealth of information collected by forensic pathologists can be effectively used in public health and safety initiatives, policies, and legislation.[20][21][28] The databases have been credited as an evidence base for awareness-raising and death-prevention initiatives informing research, policy development, and coronial investigation.[20][28] It encourages information exchange, standardization, and implementation of investigation protocols, as well as research. It has contributed to the publication of more than a hundred articles in a broad range of journals.[28][29] Access, tracking, and centralization of data can result in the improvement of scientific and investigative processes, particularly with the implementation of international standards and best practices.[21][24][28][31] The implementation of the system improved the quality of the surveillance data and the standardization of data. Furthermore, using and linking multiple sources of data enabled valuable information to be extracted and translated for the identification of vulnerable populations at risk, and it provided evidence to implement a new legislature.[21][27] It can be cost-effective and impact public health to reduce waste of resources and improve public initiatives. This data can further be used to develop diagnostic models to better inform clinical decision-making.[7] It was recognized that the tool used must be comprehensive and adaptable, as data management systems are indispensable as part of forensic investigations.[25][27] It can be remodeled to an online platform that simplifies system operations and management, improves timeliness of reporting, and increases adaptability, which in turn creates an opportunity for expansion to multiple sites.
There were several limitations discussed, which primarily involved feasibility of the system; data accuracy, availability, and completeness; involvement of relevant stakeholders; and the absence of morbidity data.[29] Collaboration may address challenges of sharing, merging, and analyzing data. Developing policies regarding storage, quality review, and access of the data for analysis may address privacy challenges. Important issues discussed included the protection of data privacy, which can be overcome by anonymization of data on the central server and storing case-sensitive information on the local server.[31][32] The use of the internet can be a cost-effective solution, whereas more sophisticated databases require time, resources, and a necessary framework that involves policies, staff, training, quality control, and support. The issues relating to the systems are the limited resources for death investigations (e.g., human factors, technology, and finances). However, the involvement of various stakeholders to support data-sharing programs in forensic pathology can relieve the financial strain.[20][21][22][23][24][25][26][27] Forensic medicine departments can utilize simple and available tools that can advance standardization of data collection, storage, and reporting because of the central role they play in reporting provincial/national data.[33] The reviewed articles included a great variety of systems that could lend themselves to use in LMIC contexts. Apart from its limitations, the autopsy/coronial data reporting systems are recognized as an essential tool for monitoring the prevalence and incidence of violence related to fatal injuries.
Strengths and limitations
This study is the first scoping review that systematically mapped literature relating to data practices in forensic medicine globally. A major strength of our study method is that it permits the inclusion of all study designs and the development of a protocol that ensures reproducibility. Moreover, we conducted a thorough search using a comprehensive search strategy which enabled us to capture the most relevant articles to answer the review question. However, the articles selected were limited to forensic medicine, keywords, and its data collection methods of autopsy records; therefore, articles that entail electronic methods of data collection in other medical departments were excluded. The limitation was due to the focus of the study being on the value of these systems to preventative programs rather than treatment. The keywords to be used in the search strategy are broad and may not identify specialized studies in data management. Only articles in English were used. Nonetheless, the findings produced by this study are useful to inform further research, particularly concerning LMICs.
Recommendations
The general consensus from the articles is that a data management and practice system containing coronial/medical examiner/forensic pathology data is beneficial for research, policymaking, prevention strategies, and information exchange for education. Due to resource limitations in some LMICs, the database can be done by using available resources to create a limited database.[33] However, if multiple stakeholders can be involved to formulate and fund a nationally representative information system, this can be wholly beneficial, not only to the community and government but may also private companies, with regard to product development and reformation due to its comprehensive coverage of preventable deaths.[20][22][24][28][29]
Conclusions
This scoping review summarized the evidence on data management and practice systems and their benefits and challenges in forensic medicine. The very appropriate use of words in an article’s title “Saving Lives Through the Power of Data” reiterates the appropriate use of information from preventable deaths. The imperative to use autopsy data for statistically relevant but also representative data can be time-consuming and an arduous task. The electronic systems, ranging from the most sophisticated (i.e., NCIS, NVDRS, Virtopsy Switzerland, Tokyo CDISC/ODM) to those created considering resource limitations (the Nigerian pilot) are cited as beneficial to the pathologist, researchers, and public health. The limitations to implementing electronic systems may include the reluctance of various stakeholders (such as government agencies) to participate and the need for additional funding to sustain more sophisticated database systems. However, the use of simple and available tools such as in the Nigerian pilot program still managed to contribute to statistically relevant data for impactful research.
Supplementary information
Additional File 1 (.docx)
Acknowledgements
Special thanks to Dr. Desmond Kuupiel who assisted in designing the database search strategy and text screening of the articles. We also thank the staff from the UKZN Medical School library for assisting in obtaining full-text articles for the review.
Author contributions
The first author conceptualized the study and prepared the scoping review under the supervision of the second author. Both first and second authors contributed to the development of the background, design of the study, and planned output of the research. The first author prepared the manuscript and the second author reviewed it. The abstract and full-text screening was conducted by the first author and an external reviewer to ensure transparency and reduce bias. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Although research ethics approval was not required for this scoping review because the study did not include human or animal participants, the study was approved by an ethics committee. Data was sourced only from published literature and gray literature.
Availability of data and materials
All data generated or analyzed during this study are included in this published scoping review article.
Competing interests
The authors declare that they have no competing interests.
Abbreviations, acronyms, and initialisms
- CADS: Central Anonymous Database System and IDS Institutional Database System (Part of CDISC/ODM Clinical Data Interchange Standards Consortium Operational Data Model)
- CDC: Centers for Disease Control and Prevention
- ICRC AM/PM Database: International Committee of the Red Cross Antemortem/Postmortem Database
- LMIC: low- to middle-income countries
- IID: Infant Injury Database
- MeSH: Medical Subject Headings
- NCIS: National Coronial Information System
- NIMSS: National Injury Mortality Surveillance System
- NVDRS: National Violent death Reporting System
- SIDS: sudden infant death syndrome
- WHO: World Health Organization
References
- ↑ Pradladh, S.; Naidoo, T. (April–June 2018). "Missed injuries in motor vehicle fatalities: Clinical vs Autopsy findings". Surgical Chronicles 23 (2): 105–111. https://www.surgchronicles.gr/index.php/en/?option=com_journals&task=byjournal&year=2018&volume=2.
- ↑ Aase, Steinar (2013). "Obduksjon – fremdeles gullstandard?" (in no). Tidsskrift for Den norske legeforening 133 (7): 730–730. doi:10.4045/tidsskr.13.0293. ISSN 0029-2001. https://tidsskriftet.no/2013/04/leder/obduksjon-fremdeles-gullstandard.
- ↑ 3.0 3.1 3.2 Bagher, A.; Wingren, C.J.; Ottosson, A.; Andersson, L.; Wangefjord, S.; Acosta, S. (1 August 2015). "Necessity of including medico-legal autopsy data in epidemiological surveys of individuals with major trauma" (in en). Injury 46 (8): 1515–1519. doi:10.1016/j.injury.2015.05.010. https://linkinghub.elsevier.com/retrieve/pii/S0020138315002685.
- ↑ Tseng, Zian H.; Olgin, Jeffrey E.; Vittinghoff, Eric; Ursell, Philip C.; Kim, Anthony S.; Sporer, Karl; Yeh, Clement; Colburn, Benjamin et al. (19 June 2018). "Prospective Countywide Surveillance and Autopsy Characterization of Sudden Cardiac Death: POST SCD Study" (in en). Circulation 137 (25): 2689–2700. doi:10.1161/CIRCULATIONAHA.117.033427. ISSN 0009-7322. PMC PMC6013842. PMID 29915095. https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.117.033427.
- ↑ 5.0 5.1 Barbería, Eneko; Gispert, Rosa; Gallo, Belén; Ribas, Gloria; Puigdefàbregas, Anna; Freitas, Adriana; Segú, Elena; Torralba, Pilar et al. (1 October 2018). "Mejora de la estadística de mortalidad por suicidio en Tarragona (Cataluña, España) entre 2004 y 2012" (in es). Revista de Psiquiatría y Salud Mental 11 (4): 227–233. doi:10.1016/j.rpsm.2016.05.004. https://linkinghub.elsevier.com/retrieve/pii/S1888989116300246.
- ↑ 6.0 6.1 Pan, Meiru; Wang, Xin; Zhao, Yunli; Liu, Wei; Xiang, Ping (1 May 2019). "A retrospective analysis of data from forensic toxicology at the Academy of Forensic Science in 2017" (in en). Forensic Science International 298: 39–47. doi:10.1016/j.forsciint.2019.02.039. https://linkinghub.elsevier.com/retrieve/pii/S0379073818309599.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 Soto Martinez, Miriam E.; Love, Jennifer C.; Pinto, Deborrah C.; Wiersema, Jason M.; Derrick, Sharon M.; Bachim, Angela; Greeley, Christopher; Donaruma‐Kwoh, Marcella et al. (1 November 2019). "The Infant Injury Database: A Tool for the Study of Injury Patterns in Medicolegal Investigations of Child Abuse" (in en). Journal of Forensic Sciences 64 (6): 1622–1632. doi:10.1111/1556-4029.14120. ISSN 0022-1198. https://onlinelibrary.wiley.com/doi/10.1111/1556-4029.14120.
- ↑ Rao, Chalapati; Lopez, Alan D.; Yang, Gonghuan; Begg, Stephen; Ma, Jiemin (1 August 2005). "Evaluating national cause-of-death statistics: principles and application to the case of China". Bulletin of the World Health Organization 83 (8): 618–625. ISSN 0042-9686. PMC 2626325. PMID 16184281. https://pubmed.ncbi.nlm.nih.gov/16184281.
- ↑ Grills, Nathan John; Ozanne-Smith, Joan; Bartolomeos, Kidist (1 June 2011). "The mortuary as a source of injury data: Progress towards a mortuary data guideline for fatal injury surveillance" (in en). International Journal of Injury Control and Safety Promotion 18 (2): 127–134. doi:10.1080/17457300.2010.540335. ISSN 1745-7300. http://www.tandfonline.com/doi/abs/10.1080/17457300.2010.540335.
- ↑ 10.0 10.1 10.2 10.3 Prinsloo, M. (2019). "Estimating injury mortality in South Africa and identifying urban-rural differences". Department of Public Health and Family Medicine, University of Cape Town. https://hdl.handle.net/11427/30083?show=full.
- ↑ 11.0 11.1 Willcox, Merlin L.; Price, Jessica; Scott, Sophie; Nicholson, Brian D.; Stuart, Beth; Roberts, Nia W.; Allott, Helen; Mubangizi, Vincent et al. (25 March 2020). "Death audits and reviews for reducing maternal, perinatal and child mortality". The Cochrane Database of Systematic Reviews 3 (3): CD012982. doi:10.1002/14651858.CD012982.pub2. ISSN 1469-493X. PMC 7093891. PMID 32212268. https://pubmed.ncbi.nlm.nih.gov/32212268.
- ↑ 12.0 12.1 12.2 Koehler, S.A. (2016). "Chapter 7: Death Investigation". In Freeman, M.; Zeegers, M.P.. Forensic epidemiology: Principles and practice. Boston, MA: Elsevier. pp. 179–199. ISBN 978-0-12-404584-2.
- ↑ Choo, Tae M.; Choi, Young-Shik (2012). "Historical Development of Forensic Pathology in the United States" (in en). Korean Journal of Legal Medicine 36 (1): 15. doi:10.7580/KoreanJLegMed.2012.36.1.15. ISSN 1225-0589. https://synapse.koreamed.org/DOIx.php?id=10.7580/KoreanJLegMed.2012.36.1.15.
- ↑ 14.0 14.1 14.2 14.3 14.4 du Toit-Prinsloo, L.; Saayman, G. (2012). "Performance of autopsies in South Africa: Selected legal and ethical perspectives". Continuing Medical Education 30 (2): 53–55. ISSN 2078-5143. http://www.cmej.org.za/index.php/cmej/article/view/2326/2188.
- ↑ 15.0 15.1 Roth, Gregory A; Abate, Degu; Abate, Kalkidan Hassen; Abay, Solomon M; Abbafati, Cristiana; Abbasi, Nooshin; Abbastabar, Hedayat; Abd-Allah, Foad et al. (1 November 2018). "Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017" (in en). The Lancet 392 (10159): 1736–1788. doi:10.1016/S0140-6736(18)32203-7. PMC PMC6227606. PMID 30496103. https://linkinghub.elsevier.com/retrieve/pii/S0140673618322037.
- ↑ Prahladh, Salona; van Wyk, Jacqueline (1 December 2020). "Protocol for a scoping review of the current data practices in forensic medicine" (in en). Systematic Reviews 9 (1): 76. doi:10.1186/s13643-020-01308-7. ISSN 2046-4053. PMC PMC7140479. PMID 32268922. https://systematicreviewsjournal.biomedcentral.com/articles/10.1186/s13643-020-01308-7.
- ↑ 17.0 17.1 Arksey, Hilary; O'Malley, Lisa (1 February 2005). "Scoping studies: towards a methodological framework" (in en). International Journal of Social Research Methodology 8 (1): 19–32. doi:10.1080/1364557032000119616. ISSN 1364-5579. http://www.tandfonline.com/doi/abs/10.1080/1364557032000119616.
- ↑ 18.0 18.1 Tricco, Andrea C.; Lillie, Erin; Zarin, Wasifa; O'Brien, Kelly K.; Colquhoun, Heather; Levac, Danielle; Moher, David; Peters, Micah D.J. et al. (2 October 2018). "PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation" (in en). Annals of Internal Medicine 169 (7): 467–473. doi:10.7326/M18-0850. ISSN 0003-4819. https://www.acpjournals.org/doi/10.7326/M18-0850.
- ↑ McGowan, Jessie; Sampson, Margaret; Salzwedel, Douglas M.; Cogo, Elise; Foerster, Vicki; Lefebvre, Carol (1 July 2016). "PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement" (in en). Journal of Clinical Epidemiology 75: 40–46. doi:10.1016/j.jclinepi.2016.01.021. https://linkinghub.elsevier.com/retrieve/pii/S0895435616000585.
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 20.10 20.11 20.12 20.13 20.14 20.15 20.16 20.17 20.18 Dunstan, Lauren (1 June 2019). "The National Coronial Information System: Saving Lives through the Power of Data" (in en). Australian Economic Review 52 (2): 247–254. doi:10.1111/1467-8462.12317. ISSN 0004-9018. https://onlinelibrary.wiley.com/doi/10.1111/1467-8462.12317.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 21.10 21.11 21.12 21.13 21.14 21.15 21.16 21.17 21.18 21.19 21.20 Fowler, Katherine A.; Jack, Shane P.D.; Lyons, Bridget H.; Betz, Carter J.; Petrosky, Emiko (2 February 2018). "Surveillance for Violent Deaths — National Violent Death Reporting System, 18 States, 2014". MMWR. Surveillance Summaries 67 (2): 1–36. doi:10.15585/mmwr.ss6702a1. ISSN 1546-0738. PMC PMC5829936. PMID 29389917. http://www.cdc.gov/mmwr/volumes/67/ss/ss6702a1.htm?s_cid=ss6702a1_w.
- ↑ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 Hargrove, Sarah L; Bunn, Terry L; Slavova, Svetla; Quesinberry, Dana; Corey, Tracey; Ralston, William; Singleton, Michael D; Ingram, Van (1 February 2018). "Establishment of a comprehensive drug overdose fatality surveillance system in Kentucky to inform drug overdose prevention policies, interventions and best practices" (in en). Injury Prevention 24 (1): 60–67. doi:10.1136/injuryprev-2016-042308. ISSN 1353-8047. https://injuryprevention.bmj.com/lookup/doi/10.1136/injuryprev-2016-042308.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 Dennis, Mark; Elder, Alexander; Semsarian, Christopher; Orchard, John; Brouwer, Isabel; Puranik, Rajesh (1 October 2018). "A 10-year review of sudden death during sporting activities" (in en). Heart Rhythm 15 (10): 1477–1483. doi:10.1016/j.hrthm.2018.04.019. https://linkinghub.elsevier.com/retrieve/pii/S1547527118303643.
- ↑ 24.00 24.01 24.02 24.03 24.04 24.05 24.06 24.07 24.08 24.09 24.10 24.11 24.12 24.13 24.14 24.15 24.16 24.17 24.18 24.19 24.20 Saar, Eva; Bugeja, Lyndal; Ranson, David L. (1 December 2017). "National Coronial Information System: Epidemiology and the Coroner in Australia" (in en). Academic Forensic Pathology 7 (4): 582–590. doi:10.23907/2017.049. ISSN 1925-3621. PMC PMC6474448. PMID 31240008. http://journals.sagepub.com/doi/10.23907/2017.049.
- ↑ 25.00 25.01 25.02 25.03 25.04 25.05 25.06 25.07 25.08 25.09 25.10 25.11 25.12 Hofmeister, Ute; Martin, Shuala S.; Villalobos, Carlos; Padilla, Juliana; Finegan, Oran (1 October 2017). "The ICRC AM/PM Database: Challenges in forensic data management in the humanitarian sphere" (in en). Forensic Science International 279: 1–7. doi:10.1016/j.forsciint.2017.07.022. https://linkinghub.elsevier.com/retrieve/pii/S0379073817302815.
- ↑ 26.00 26.01 26.02 26.03 26.04 26.05 26.06 26.07 26.08 26.09 26.10 Ottaviani, Giulia; Perlasca, Paolo; Mesiti, Marco; Ferrari, Luca; Lavezzi, Anna M. (1 July 2017). "Authorised access web portal for Italian data bank on sudden unexpected perinatal and infant death" (in en). Acta Paediatrica 106 (7): 1196–1197. doi:10.1111/apa.13833. https://onlinelibrary.wiley.com/doi/10.1111/apa.13833.
- ↑ 27.00 27.01 27.02 27.03 27.04 27.05 27.06 27.07 27.08 27.09 27.10 27.11 27.12 27.13 27.14 Levy, Bruce (1 January 2015). "The need for informatics to support forensic pathology and death investigation" (in en). Journal of Pathology Informatics 6 (1): 32. doi:10.4103/2153-3539.158907. PMC PMC4485186. PMID 26167376. https://linkinghub.elsevier.com/retrieve/pii/S2153353922004874.
- ↑ 28.00 28.01 28.02 28.03 28.04 28.05 28.06 28.07 28.08 28.09 28.10 28.11 28.12 28.13 28.14 28.15 28.16 28.17 28.18 28.19 28.20 28.21 28.22 28.23 28.24 28.25 Blair, Janet M; Fowler, Katherine A; Jack, Shane P D; Crosby, Alexander E (1 April 2016). "The National Violent Death Reporting System: overview and future directions" (in en). Injury Prevention 22 (Suppl 1): i6–i11. doi:10.1136/injuryprev-2015-041819. ISSN 1353-8047. PMC PMC6049659. PMID 26718549. https://injuryprevention.bmj.com/lookup/doi/10.1136/injuryprev-2015-041819.
- ↑ 29.00 29.01 29.02 29.03 29.04 29.05 29.06 29.07 29.08 29.09 29.10 29.11 29.12 29.13 29.14 29.15 29.16 29.17 29.18 29.19 29.20 Bugeja, Lyndal; Ibrahim, Joseph E.; Ferrah, Noha; Murphy, Briony; Willoughby, Melissa; Ranson, David (1 December 2016). "The utility of medico-legal databases for public health research: a systematic review of peer-reviewed publications using the National Coronial Information System" (in en). Health Research Policy and Systems 14 (1): 28. doi:10.1186/s12961-016-0096-1. ISSN 1478-4505. PMC PMC4828834. PMID 27067413. http://health-policy-systems.biomedcentral.com/articles/10.1186/s12961-016-0096-1.
- ↑ 30.0 30.1 30.2 30.3 30.4 Pearse, J (1 October 2012). "THE NATIONAL CORONIAL INFORMATION SYSTEM: A DECADE OF CHALLENGES AND ACHIEVEMENTS" (in en). Injury Prevention 18 (Suppl 1): A22.1–A22. doi:10.1136/injuryprev-2012-040580b.24. ISSN 1353-8047. https://injuryprevention.bmj.com/lookup/doi/10.1136/injuryprev-2012-040580b.24.
- ↑ 31.00 31.01 31.02 31.03 31.04 31.05 31.06 31.07 31.08 31.09 31.10 31.11 Kiuchi, Takahiro; Yoshida, Ken-ichi; Kotani, Hirokazu; Tamaki, Keiji; Nagai, Hisashi; Harada, Kazuki; Ishikawa, Hirono (1 November 2013). "Legal Medicine Information System using CDISC ODM" (in en). Legal Medicine 15 (6): 332–334. doi:10.1016/j.legalmed.2013.08.003. https://linkinghub.elsevier.com/retrieve/pii/S1344622313000916.
- ↑ 32.00 32.01 32.02 32.03 32.04 32.05 32.06 32.07 32.08 32.09 32.10 32.11 32.12 Aghayev, Emin; Staub, Lukas; Dirnhofer, Richard; Ambrose, Tony; Jackowski, Christian; Yen, Kathrin; Bolliger, Stephan; Christe, Andreas et al. (1 April 2008). "Virtopsy – The concept of a centralized database in forensic medicine for analysis and comparison of radiological and autopsy data" (in en). Journal of Forensic and Legal Medicine 15 (3): 135–140. doi:10.1016/j.jflm.2007.07.005. https://linkinghub.elsevier.com/retrieve/pii/S1752928X07001059.
- ↑ 33.00 33.01 33.02 33.03 33.04 33.05 33.06 33.07 33.08 33.09 33.10 33.11 Kipsaina, Chebiwot; Eze, Uwom O.; Ozanne-Smith, Joan (3 July 2015). "A standardised mortuary-based injury surveillance system: lessons learned from the Ibadan Nigerian trial" (in en). International Journal of Injury Control and Safety Promotion 22 (3): 193–202. doi:10.1080/17457300.2014.884142. ISSN 1745-7300. http://www.tandfonline.com/doi/full/10.1080/17457300.2014.884142.
- ↑ 34.00 34.01 34.02 34.03 34.04 34.05 34.06 34.07 34.08 34.09 34.10 34.11 Colville-Ebeling, Bonnie; Frisch, Morten; Lynnerup, Niels; Theilade, Peter (1 November 2014). "HOMED—Homicides Eastern Denmark: An introduction to a forensic medical homicide database" (in en). Scandinavian Journal of Public Health 42 (7): 683–686. doi:10.1177/1403494814544402. ISSN 1403-4948. http://journals.sagepub.com/doi/10.1177/1403494814544402.
- ↑ 35.00 35.01 35.02 35.03 35.04 35.05 35.06 35.07 35.08 35.09 35.10 Statistics Canada, Public Health Agency of Canada (February 2012). "Canadian Coroner and Medical Examiner Database: Annual Report, 2006–2008". Minister of Industry. ISSN 1927-775X. https://www150.statcan.gc.ca/n1/en/catalogue/82-214-X2012001.
- ↑ Centers for Disease Control and Prevention (18 April 2024). "National Violent Death Reporting System (NVDRS)". U.S. Department of Health & Human Services. https://www.cdc.gov/violenceprevention/datasources/nvdrs/index.html.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation, grammar, and punctuation. In some cases important information was missing from the references, and that information was added. The original article lists references in alphabetical order; this version lists them in order of appearance, by design.