Fertilization following pollination predominantly decreases phytocannabinoids accumulation and alters the accumulation of terpenoids in Cannabis inflorescences
| Full article title | Fertilization following pollination predominantly decreases phytocannabinoids accumulation and alters the accumulation of terpenoids in Cannabis inflorescences |
|---|---|
| Journal | Frontiers in Plant Science |
| Author(s) | Feder, Carni L.; Cohen, Oded; Shapira, Anna; Katzir, Itay; Peer, Reut; Guberman, Ohad; Procaccia, Shiri; Berman, Paula; Flaishman, Moshe; Meiri, David |
| Author affiliation(s) | Technion-Israel Institute of Technology, Agricultural Research Organization of Israel |
| Primary contact | Email: dmeiri at technion dot ac dot il |
| Editors | Taglialatela-Scafati, Orazio |
| Year published | 2021 |
| Volume and issue | 12 |
| Article # | 753847 |
| DOI | 10.3389/fpls.2021.753847 |
| ISSN | 1664-462X |
| Distribution license | Creative Commons Attribution 4.0 International |
| Website | https://www.frontiersin.org/articles/10.3389/fpls.2021.753847/full |
| Download | https://www.frontiersin.org/articles/10.3389/fpls.2021.753847/pdf (PDF) |
Abstract
Over the last few decades, a growing body of evidence has increasingly showed the therapeutic capabilities of Cannabis plants. These capabilities have been attributed to the specialized secondary metabolites stored in the glandular trichomes of female inflorescences, mainly phytocannabinoids and terpenoids. The accumulation of these metabolites in the flower is versatile and influenced by a largely unknown regulation system, attributed to genetic, developmental, and environmental factors. As Cannabis is a dioecious plant, one main factor is fertilization after successful pollination. Fertilized flowers are considerably less potent, likely due to changes in the contents of phytocannabinoids and terpenoids.
This study examined the effect of fertilization on metabolite composition by crossbreeding Δ9-tetrahydrocannabinol (Δ9-THC)- or cannabidiol (CBD)-rich female plants with different male plants: THC-rich plants, CBD-rich plants, or the original female plant induced to develop male pollen sacs. We used advanced analytical methods to assess the phytocannabinoid and terpenoid content, including a newly developed semi-quantitative analysis for terpenoids without analytical standards.
We found that fertilization significantly decreased phytocannabinoid content. For terpenoids, the subgroup of monoterpenoids had similar trends to the phytocannabinoids, proposing both are commonly regulated in the plant. The sesquiterpenoids remained unchanged in the THC-rich female plants and had a trend of decreasing in the CBD-rich female plants. Additionally, specific phytocannabinoids and terpenoids showed an uncommon increase in concentration following fertilization with particular male plants.
Our results demonstrate that although the profile of phytocannabinoids and their relative ratios were kept, fertilization substantially decreased the concentration of nearly all phytocannabinoids in the plant regardless of the type of fertilizing male plant. Our findings may point to the functional roles of secondary metabolites in Cannabis.
Keywords: Cannabis, cannabinoids, terpenoids, secondary metabolites, chromatography/mass spectrometry, analytical methods, gas chromatography, high pressure liquid chromatography
Introduction
Cannabis sativa L. (Cannabis) has been known as a medicinal plant since ancient times.[1] During the last two decades, many studies have added to the growing evidence for its therapeutic effects in a wide range of conditions such as neurodegenerative disorders[2][3], pain[4], epilepsy,[5] multiple sclerosis[6], and more.[7] These therapeutic abilities have been attributed to the secondary metabolites biosynthesized in Cannabis[8], with more than 500 different secondary metabolites having been identified.[9][10] These metabolites belong to several groups of compounds, including phytocannabinoids, terpenoids, and flavonoids.
To date, the most characterized are phytocannabinoids, lipophilic compounds made of isoprene units (five-carbon building blocks)[11], which are almost exclusive to the Cannabis plant.[12] More than 140 different phytocannabinoids have been found to accumulate to various extents in glandular trichomes that are located in the aerial parts of the plant and mostly on the female flowers, which are arranged in a cluster on the stem of the inflorescence.[11] Phytocannabinoids can be classified into several subclasses according to their chemical structure, including the Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) families, as well as cannabinol (CBN), cannabigerol (CBG), cannabichromene (CBC), and many others.[10][13]
Terpenoids, found in many other plants, represent a second large group of metabolites. These metabolites are closely related to phytocannabinoids, sharing the same isoprenoid precursor and built up by branched isoprene units.[14] Terpenoids are responsible for the fragrance and taste of the plant and are suggested to also have defensive roles. They may also contribute to the therapeutic effects attributed to Cannabis.[15]
Another group of metabolites worth mentioning is flavonoids. Among this group, which is widespread in the plant kingdom, there are three specific prenylated flavonoids, termed Cannflavins A, B, and C, which are unique to Cannabis and show potent anti-inflammatory abilities.[16][17][18]
Ongoing research is focused on matching specific metabolites found in the plant and their therapeutic capabilities. To this end, specialized analytical methods have been developed in order to obtain precise knowledge on all the components of the plant and the effects they are responsible for. Currently, more than 90 phytocannabinoids and 100 terpenoids are routinely identified and quantified to obtain an overall chemical profile of each chemovar used for a medicinal purpose.[13][19] In parallel to the search for specific biological activities of the secondary metabolites, broad ongoing research is focused upon the elucidation of in planta metabolites’ biosynthesis, transport, and accumulation pathways. Genome, transcriptome, and proteome data have been published since 2011[20][21][22][23][24], and have been integrated into a genomic database for Cannabis (CannabisGDB).[25] Biosynthetic pathways are being unraveled, and recently more than 30 Cannabis-specific terpenoid synthases have been characterized.[14][23][26][27][28] In addition, the environmental and developmental factors that affect metabolite accumulation are also studied, such as light levels[29][30][31], soil types, and harvest time.[32][33][34] The increasing information on the impact of these different factors on metabolite accumulation has the prospect of developing specific chemovars harboring a pre-planned group of metabolites.[35]
This study examined the effect of an additional factor, the fertilization of Cannabis flowers following pollination of the pistil. Fertilization of flowers is a key step in the plant life cycle. Successful pollination activates a series of events followed by fertilization and embryogenesis. This includes the development of an ovary on one hand, together with senescence and abscission of floral organs, degradation of macromolecules, and recycling of different nutrients on the other hand.[36][37][38] Cannabis is a dioecious plant, harboring either female or male reproductive organs. It is also a wind-pollinated plant, in which the pollination of flowers is not dependent on specific animal pollinators. Phytocannabinoids are most abundant in the female flower inflorescences.[10] Fertilized flowers, harboring seeds, are considerably less potent. Hence the term “sinsemilla,” Spanish for “without seed,” that defines plants associated with high psychoactive effects.[39] In addition, it is a common work practice by Cannabis growers to eliminate male plants growing in a field to maintain the unfertilized inflorescences and maximize the phytocannabinoid concentrations. Therefore, it is likely that the content of secondary metabolites such as phytocannabinoids and terpenoids changes following the pollination and fertilization of Cannabis inflorescences. However, although mentioned in a few studies[15][32][40], this phenomenon was not studied in depth. In the last few years, an increasing number of Cannabis growers are moving from using cuttings from female “mother plants” to seeds. Even though the seeds are usually feminized, around 5–10% will be males, and thus the question about the effect of pollination on the phytocannabinoids and terpenoids expression becomes critical.
In order to gain more insight into the Cannabis metabolite regulation pathway, this work studied the effect of flower fertilization on the plant’s secondary metabolite accumulation. We used indoor growing methods together with analytical procedures in order to investigate the effect of fertilization on metabolite composition and concentration in Cannabis inflorescences, and specify which metabolites are affected and to what extent.
Materials and methods
Chemicals and reagents
Liquid chromatography–mass spectrometry (LC/MS)-grade acetonitrile (catalog number 1.00029), methanol (1.06035), and water (1.15333); and gas chromatography (GC) headspace-grade dimethyl sulfoxide (DMSO) (1.01900) were purchased from Mercury Scientific and Industrial Products Ltd. (Rosh Haayin, Israel). Ethanol, (catalog number 052541), acetic acid (010778) and n-Hexane (091484) were obtained from BioLab Ltd. (Jerusalem, Israel). Phytocannabinoid analytical standards (>98%) CBG, THC, CBD, CBC, CBN, cannabigerolic acid (CBGA), tetrahydrocannabinolic acid (THCA), cannabidiolic acid (CBDA), cannabinolic acid (CBNA), cannabichromenic acid (CBCA), Δ8-tetrahydrocannabinol (Δ8-THC), tetrahydrocannabivarin (THCV), cannabidivarin (CBDV), cannabidivarinic acid (CBDVA), and cannabicyclol (CBL) were purchased from Sigma-Aldrich (Rehovot, Israel); cannabichromevarin (CBCV) was purchased from Cayman Chemical (Ann Arbor, MI, United States). Terpenoid analytical standards (>95% unless stated otherwise) were purchased from Sigma-Aldrich (Rehovot, Israel); valencene (>80% pure), α- and β-curcumene (>90% pure), α-phellandrene, and sabinene were purchased from Extrasynthese (Genay, France); a mixture of n-alkanes was purchased from Sigma R 769 (40 mg/mL, C8-C20, Saint Louis, MO, United States) for semi-quantitative analysis.
Experimental design
The effect of fertilization was tested on two Cannabis sativa L. female plants. Female strains 333 THC-rich (15% THCA, 0.07% CBDA) and 423 CBD-rich (0.33% THCA, 9% CBDA) were subjected to fertilization, and two male plants strains—319 THC-rich (progeny of high THC landrace Highland Thai, Seedsman seeds) and 405 CBD-rich (progeny of Cherry CBD)—were used as pollen donors. In addition, the female plants were subjected to a sex conversion treatment[41][42], and these induced males were also used as pollen donors to fertilize the two female plants. In order to achieve pollen sacs, 45-days old rooted cutting, 30 cm size female plants were sprayed daily until completely moist with ethylene inhibitor (Sodium Thiosulfate 0.5%) for five days prior to transferring to short day conditions. The female plants that were sex changed are referred to as males or induced-males. The female Cannabis plants were grown, three plants for each treatment, under an 18/6 light/dark regime (800 μmol), 23–27°C for 30 days before being transferred to flowering chambers with a 12/12 light/dark regime (500 μmol), 23–27°C for up to either 42 (six weeks) or 56 (eight weeks) days before some inflorescences were removed for chemical analysis. Female plants were grown in small flowering chambers (1 m2) in the presence of a single pollen donor. All plants were grown in 1 L pots on a mixture of pit/coconut 70%/30% soil, respectively. l The inflorescences of the treated plants, averaging 3–4 apical inflorescences per plant, were harvested and dried for 24–48 hours at 40°C until they reached a moisture content of 12% weight for weight (w/w). The inflorescences were ground to a fine powder using an electric grinder, then 98–103 mg were weighed and extracted with 1 mL ethanol. Samples were sonicated in an ultrasonic bath for 30 minutes, agitated in an orbital shaker at 25°C for 20 minutes, centrifuged at 20,000 x g for 5 minutes, then the samples were dissolved and diluted x20 in ethanol and filtered through a 0.22 μm Polytetrafluoroethylene syringe filter (Lumitron Ltd., Petah Tikva, Israel) prior to analysis.
Phytocannabinoid identification and quantification
Phytocannabinoid analyses for high concentrations of THC and CBD were performed using a Thermo Scientific UltiMate 3000 ultra-high-performance liquid chromatography coupled with an ultraviolet-visible diode array detector (UHPLC/UV) system. All other phytocannabinoids were identified and quantified by a similar UHPLC instrument coupled with a Q Exactive Focus Hybrid Quadrupole-Orbitrap MS (Thermo Scientific, Bremen, Germany), as previously described by Berman et al.[13] and Milay et al.[43] In short, chromatographic separation was achieved using a HALO C18 Fused-Core column (2.7 μm, 150 × 2.1 mm), with a HALO guard column (2.7 μm, 5 × 2.1 mm), and a ternary A/B/C multistep gradient (solvent A: water with 0.1% acetic acid; solvent B: acetonitrile with 0.1% acetic acid,; and solvent C: methanol). Identification and absolute quantification of phytocannabinoids were performed by external calibrations, as previously described by Berman et al.[13] Sixteen analytical standards (CBDVA, CBDA, CBCA, CBNA, CBGA, THCA, CBDV, CBD, CBC, CBN, CBG, THC, Δ8-THC, CBL, THCV, CBCV) were used for direct quantification and semi-quantification of additional phytocannabinoids. All extracted samples were injected and analyzed by an electrospray ionization (ESI)-LC/MS analysis, diluted at ratios of 1:9, 1:99, and 1:999 v/v Cannabis extract to ethanol.
Terpenoids identification and quantification
Profiling of terpenoids was performed using a modification of the static headspace gas chromatography–tandem mass spectrometry (SHS-GC/MS/MS) method by full evaporation technique.[19] SHS-injections were performed by PAL RTC robotic tool (CTC Analytics, Swaziland) with 30 minute incubation time, temperature of 140°C, and 1,000 μL injection volume of the gas phase. Gas chromatographic separation was achieved in 74 minutes using a TRACE 1310 GC (Thermo Fisher Scientific, Bremen, Germany) equipped with a 30 m × 0.25 mm × 0.25 μm capillary DB-35MS UI column (Agilent Technologies, United States). MS/MS compound detection was performed by a TSQ 8000 Evo triple quadrupole mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). For terpenoids analyses, 10 mg of each ground Cannabis sample was weighed in duplicates in a 20 mL HS amber vial with 1.2 g of glycerol and sealed by a magnetic cap. Solutions for the construction of the calibration curves were prepared in hexane, and then 10 μL for each calibration level was added to amber vials with 1.2 g of glycerol in the same manner as the samples.
Some of the terpenoids were calculated semi-quantitatively based on the calibration curves of terpenoids with commercially available analytical standards with similar MS spectral characteristics and retention times. Identification of these terpenoids was performed by spectral searching against the NIST library (version 2.2) and relative Kovats retention indices using a mixture of n-alkanes run under the same chromatographic conditions (for full details, see Supplementary material, Tables 2 and 3).
Statistical analysis
Statistical analyses were conducted using GraphPad Prism software version 8.2.1 (GraphPad Inc.). Differences between samples in phytocannabinoid and terpenoid concentrations were analyzed using two-way ANOVA followed by Dunnett’s multiple comparison test. P-values were corrected for multiple testing using the Tukey post hoc test. A value of at least p ≤ 0.05 was considered significant for all tests (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001). Outliers were defined as data points greater than two standard deviations from the mean (9.6 for THCA and 9.5 for CBDA).
Results
Phytocannabinoids quantity predominantly decreases after fertilization
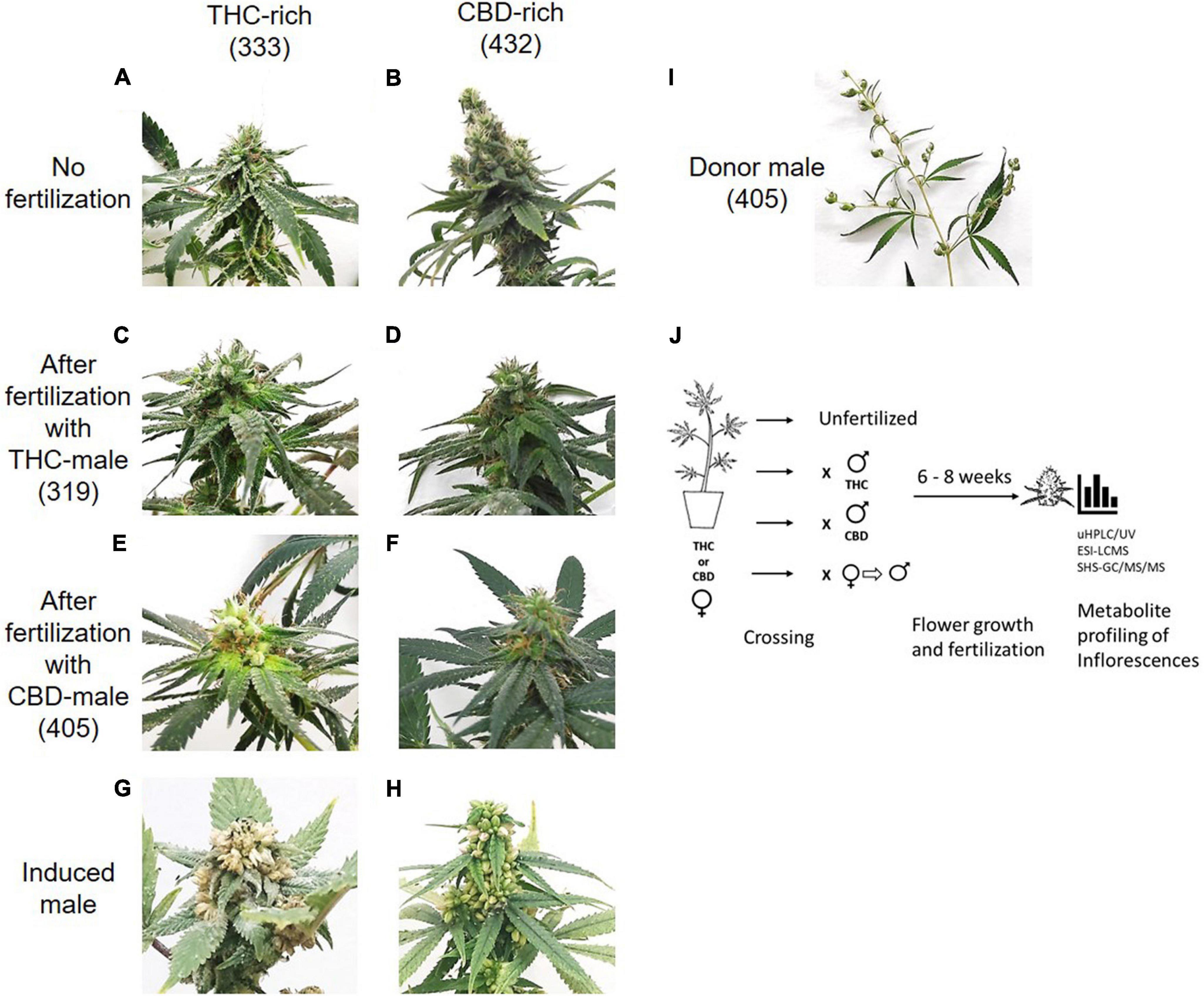
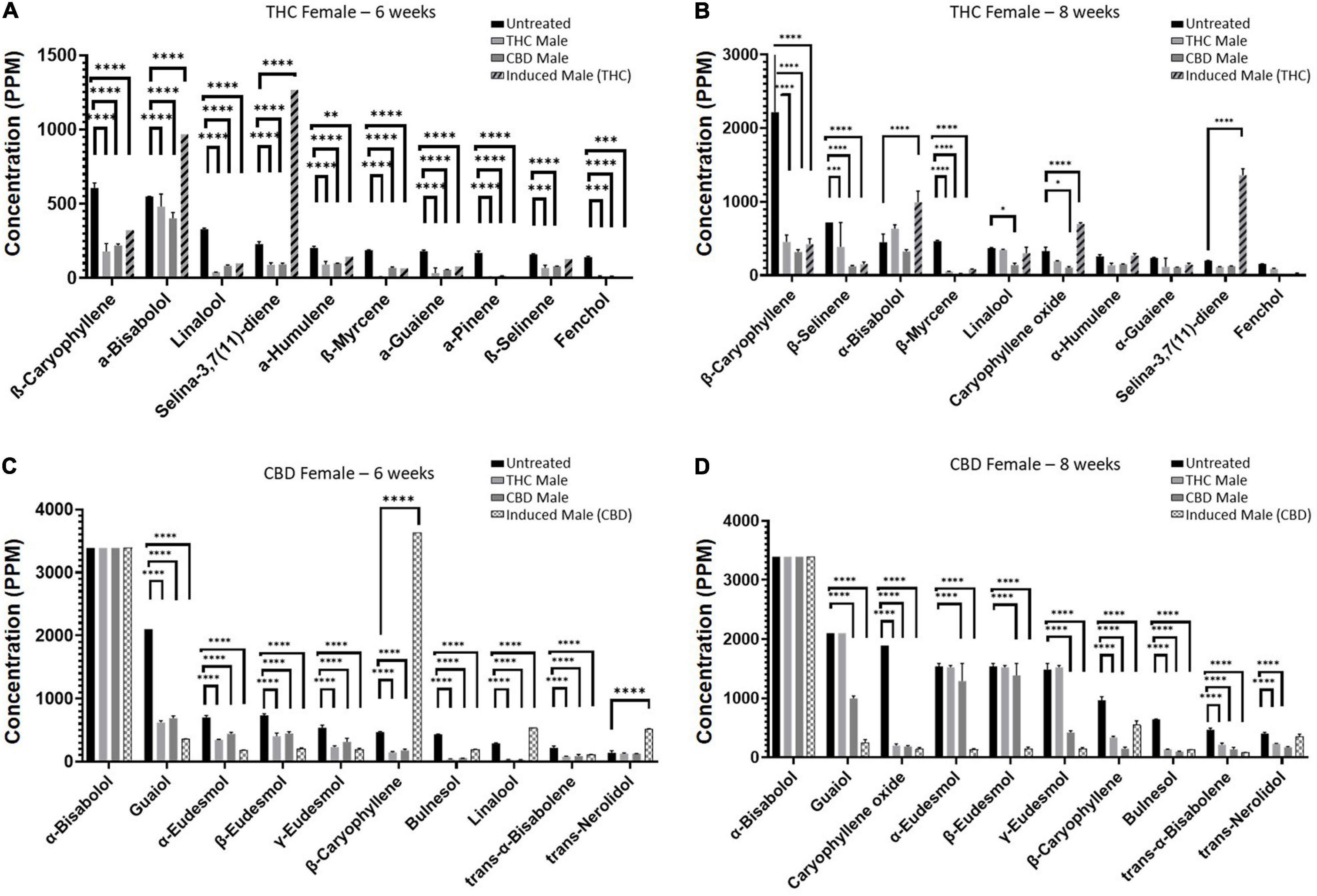
Mature inflorescences (six or eight weeks post-flower-induction) from female Cannabis plants of two distinct chemovars (Figures 1A and B), THC-rich (Type I) and CBD-rich (Type III), were subjected to fertilization by three different male Cannabis plant types: THC-rich (Figures 1C and D), CBD-rich (Figures 1E and F), or the original female plant induced to develop male pollen sacs by application of ethylene inhibitor (Figure 1). Induced-male plants (Figures 1G and H) were genetically identical to the female plants, and they had a distinct change in the sex of the flowers after treatment and a larger number of inflorescences compared to males (Figure 1I). Specific fertilization was achieved by incubation of the individual plants. (Figure 1J)
|
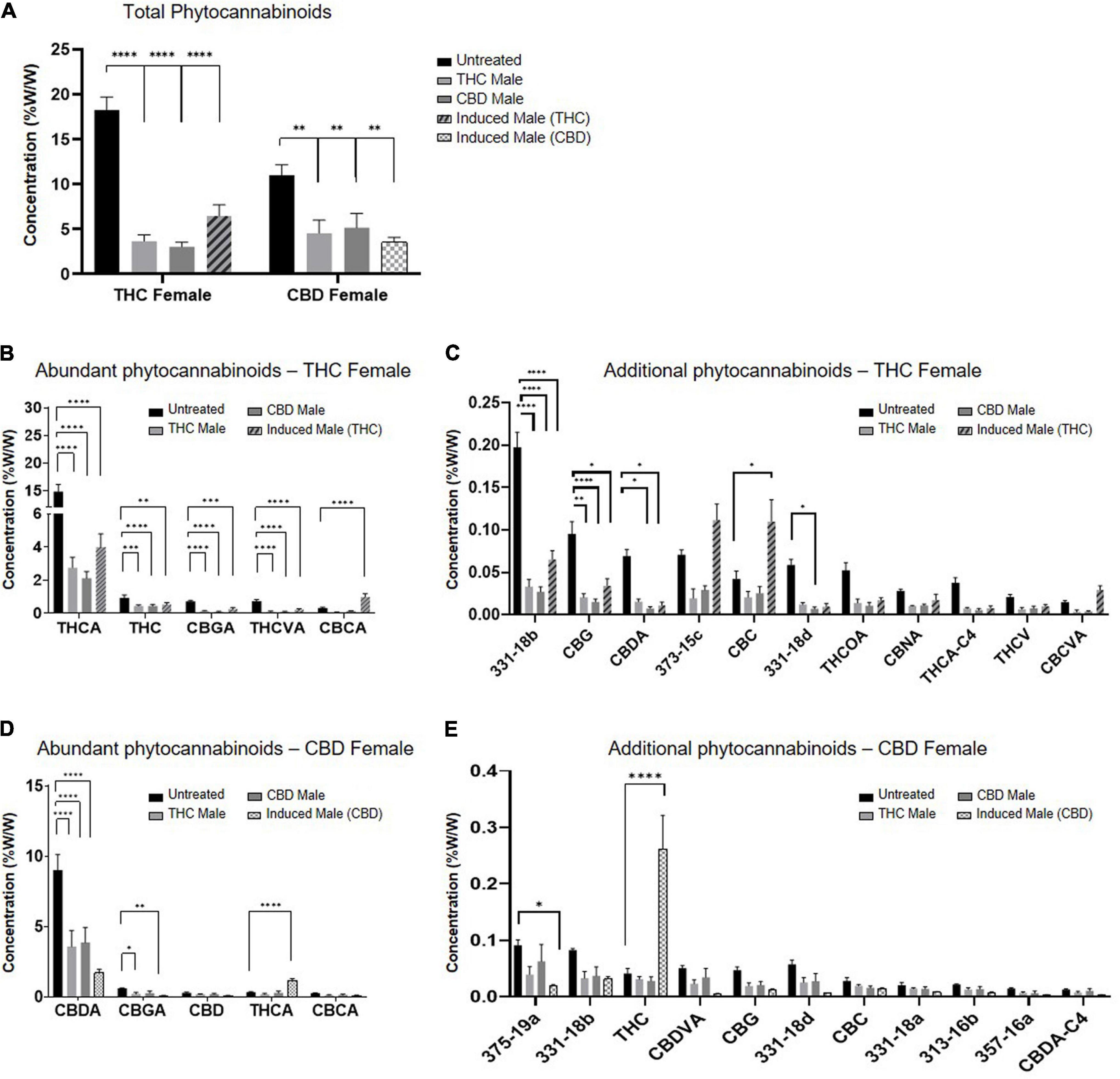
Fertilization resulted in a predominantly significant decrease of overall total phytocannabinoids concentration in inflorescences for both the THC-rich and CBD-rich females, by all three types of males (Figure 2A). The concentration of the phytocannabinoids was analyzed by UHPLC/UV and ESI-LC/MS. (The full list of the 95 phytocannabinoids quantified, as named by Berman et al.[13], is displayed in Supplementary material, Table 1.) A sharper decrease was detected in the THC-rich chemovar female, exhibiting an average 75% decrease, while CBD-rich females showed a 60% decrease in phytocannabinoid contents after fertilization. Next, we investigated changes in quantities of individual phytocannabinoids (Figures 2B–E). For the THC-female, fertilization caused a reduction in the abundant phytocannabinoids, whose concentrations in the plant were above 0.02%, except for the phytocannabinoid CBCA, which had an increase of about 50% when the plant was fertilized with an induced male (Figure 2B). Additional phytocannabinoids, whose concentrations in the plant were 0.001–0.2%, were also mostly reduced upon fertilization. The concentrations of CBC, cannabichromevarinic acid (CBCVA), and 373-15c were increased when fertilized by the induced male (Figure 2C). When THCA was excluded as an outlier, as its concentration is 15-fold higher, the less abundant phytocannabinoids 331-18b, CBG, CBDA, and 331-18d were significantly reduced upon fertilization. Similarly, for the CBD-female, fertilization caused a reduction in both the abundant (Figure 2D) and additional phytocannabinoids (when CBDA is excluded as an outlier) (Figure 2E), except for the concentrations of THCA and THC that increased after fertilization with the induced male.
|
Terpenoids quantity decreases after fertilization in the cannabidiol-rich female plant but varies in the THC-rich female plant
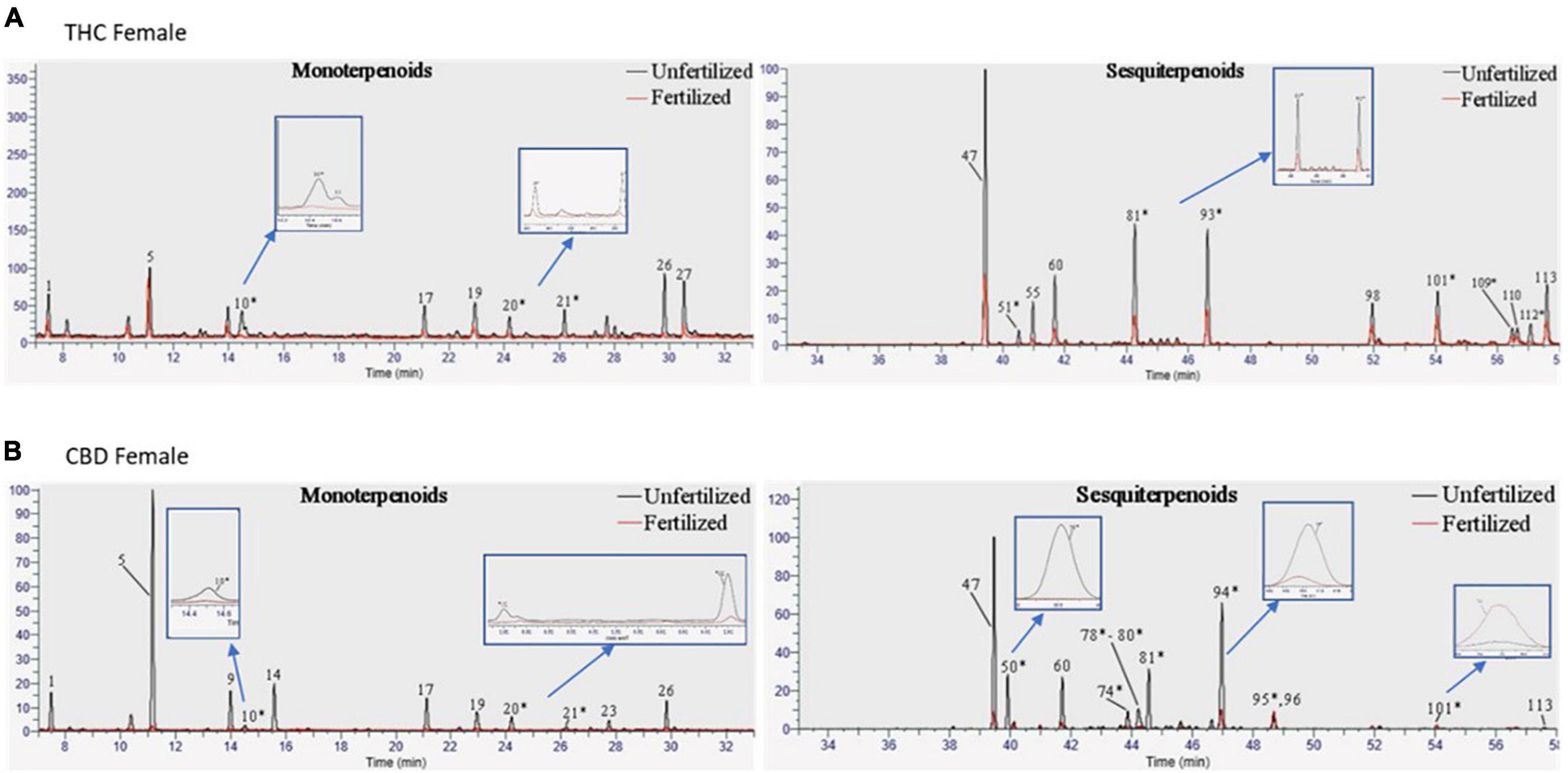
In addition to assessing the phytocannabinoid contents, we quantified over 100 terpenoid compounds. The THC- and CBD-rich female plants differed in their profile of terpenoids before fertilization (Supplementary material, Figure 1). About half of the quantified terpenoids had pure analytical standards available and were analyzed as previously described.[19] However, out of a total of 113 terpenoids detected using (SHS-GC/MS/MS), 63 terpenoids in either the THC-rich or the CBD-rich plants did not have commercially available standards (for a full list, see Supplementary material, Table 4). Some of these terpenoids demonstrated significant changes after fertilization, therefore, we assessed them with a newly developed semi-quantitative analysis (Figure 3). In this manner, we quantified terpenoids such as δ-guaiene and trans-α-bisabolene (denoted as 81 and 93, respectively). The semi-quantitative analysis is based on the calibration curves of terpenoids with commercially available analytical standards, relying primarily on similar MS spectral characteristics and also on retention times (Supplementary material, Figure 2).
|
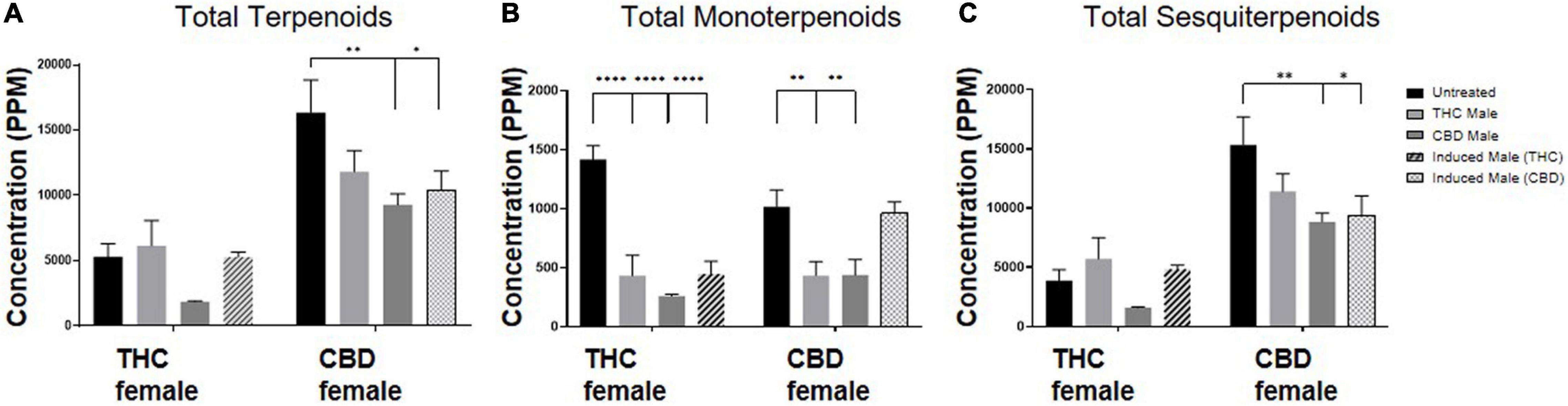
The total amount of terpenoids in the inflorescences was found to be chemovar-specific (Figure 4A). The high-CBD female plants exhibited two to threefold higher concentrations of terpenoids, both in the unfertilized and all three types of fertilized plants, compared to the THC-rich female plants. Upon fertilization, there were no significant changes in terpenoid accumulation in the THC-rich female. In the CBD-rich female plants, there was no significant change when fertilized with a THC-rich male plant, but fertilization with a CBD-rich male or an induced male resulted in a significant reduction in total terpenoids. The profile of terpenoids in plants is highly variable[14], and being mostly volatile compounds, they are also more susceptible to changes due to sample preparation procedure, e.g., the freshness of samples.[44] We detected an overall fertilization-dependent decrease in total terpenoid accumulation only in the CBD-rich plant, while the THC-rich plant showed a mixed trend of changes, either reduction or no significant change.
|
Out of 113 terpenoids detected, 31 were monoterpenoids, built up by two isoprene units (10 carbons), and the rest were sesquiterpenoids, built up by three isoprene units (15 carbons).[19] To further evaluate the influence of fertilization on terpenoid accumulation after fertilization, we analyzed these two distinct subgroups. Monoterpenoid concentrations were significantly reduced for the THC-rich female by 60–80% upon fertilization with all three types of male plants; for the CBD-rich female, there was a significant 50% reduction except for when fertilized by the induced-male, which left the concentrations unchanged (Figure 4B). The concentration of sesquiterpenoids was unchanged for the THC-rich female, but there was a trend of reduced concentrations in the CBD-rich fertilized female, which was statistically significant when fertilized with the CBD-rich or the induced male (Figure 4C).
Individual terpenoid concentrations are differentially affected by fertilization
Next, we set out to examine the accumulation of individual terpenoids in the plants (Figure 5) and found chemovar-specific differences. For the THC-rich female, the most abundant terpenoid was β-caryophyllene, and its concentration was reduced upon fertilization (Figures 5A,B). For the CBD-rich female, the most abundant terpenoid was α-bisabolol, its concentration was above detection limit both before and after fertilization (Figures 5C,D). Moreover, we noticed that the terpenoid profile changed during the length of the flowering time, between six to eight weeks after fertilization. This was in contrast to the phytocannabinoids profile, which was more consistent between these two time-points (data not shown). For example, for the CBD-rich female, the sesquiterpenoid caryophyllene oxide had a very low concentration in the six-week flowering plant but became highly abundant in the eight-week plant (Figures 5C,D). Hence, in addition to chemovar-specific differences, differential accumulation was observed between six- and eight-week growth in the same chemovar.
|
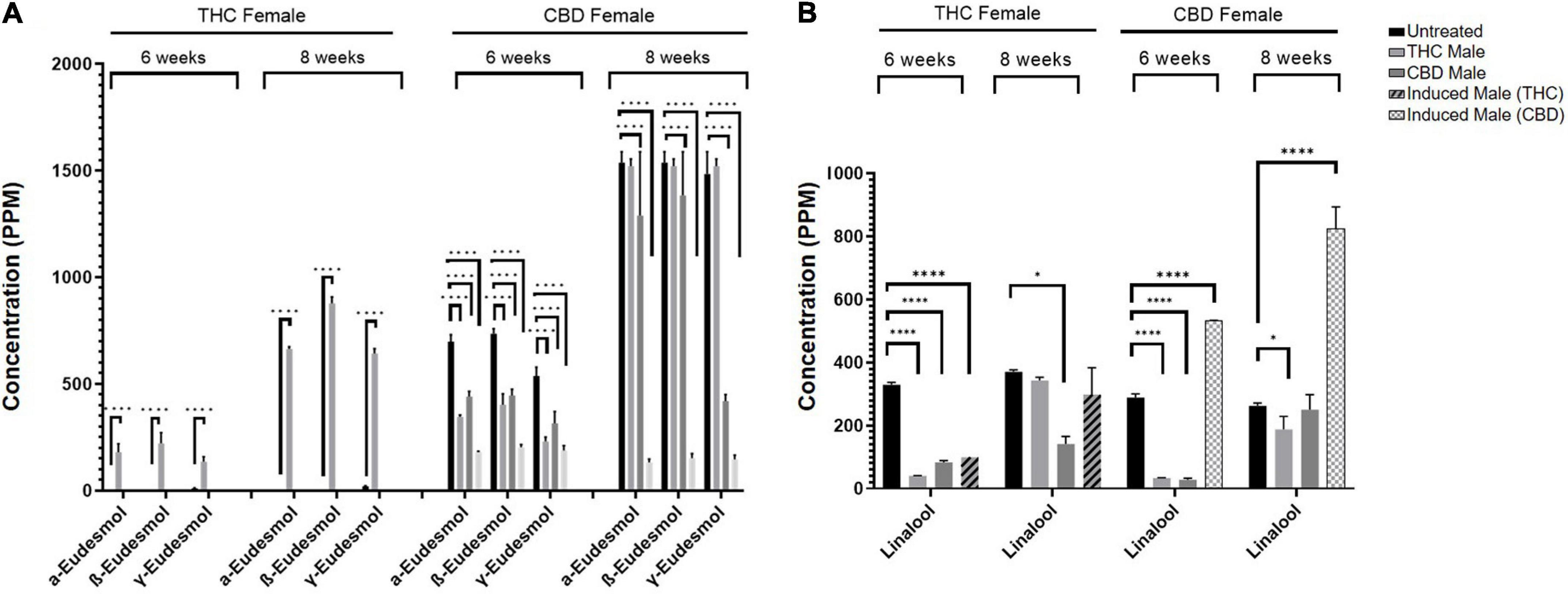
As seen in Figure 5, numerous terpenoids significantly decreased following fertilization. However, several specific terpenoids showed an interesting increase in concentration after fertilization. For example, in the THC-rich female, members of the eudesmol family of sesquiterpenoids (α-, β-, and γ-eudesmol) were mostly undetected in the unfertilized plant, but their concentrations were significantly increased upon fertilization by the THC male plant only, both at six and eight weeks after fertilization (Figure 6A). Interestingly, the levels of these terpenoids were either reduced or unchanged in the CBD-rich female due to fertilization processes. In contrast, in the CBD-rich female, the monoterpenoid linalool significantly increased upon fertilization by the induced male, but was reduced or unchanged following all other fertilization processes in both plant chemovars (Figure 6B).
|
Discussion
The present study was designed to examine the influence of flower fertilization on the accumulation of Cannabis secondary metabolites. The primary outcome is the significant overall decrease in phytocannabinoid metabolites upon fertilization. This decrease was evident in almost all phytocannabinoids measured, regardless if those were the abundant ones or the relatively low accumulating components (Figure 2). Though the altogether amount of phytocannabinoids is drastically reduced, the ratio between the different compounds is kept and their profile in the plant remains principally unchanged.
Terpenoid concentrations mostly decreased but varied. While monoterpenoids had a similar decrease as portrayed by the phytocannabinoids, sesquiterpenoids exhibited a more diverse pattern, some of which increased and some decreased upon fertilization (Figure 3). However, examining specific metabolites can point to several phytocannabinoids or terpenoids that have an individual trend, suggesting a more complex regulatory network (Figures 4 and 5).
First, these results confirm that when the objective is to maintain high levels of phytocannabinoids, fertilization must be avoided. Apart from a physical separation between female and male flowers or vegetative reproduction, this goal could be achieved using advanced genetic manipulations that target female fertilization pathways.[45][46][47]
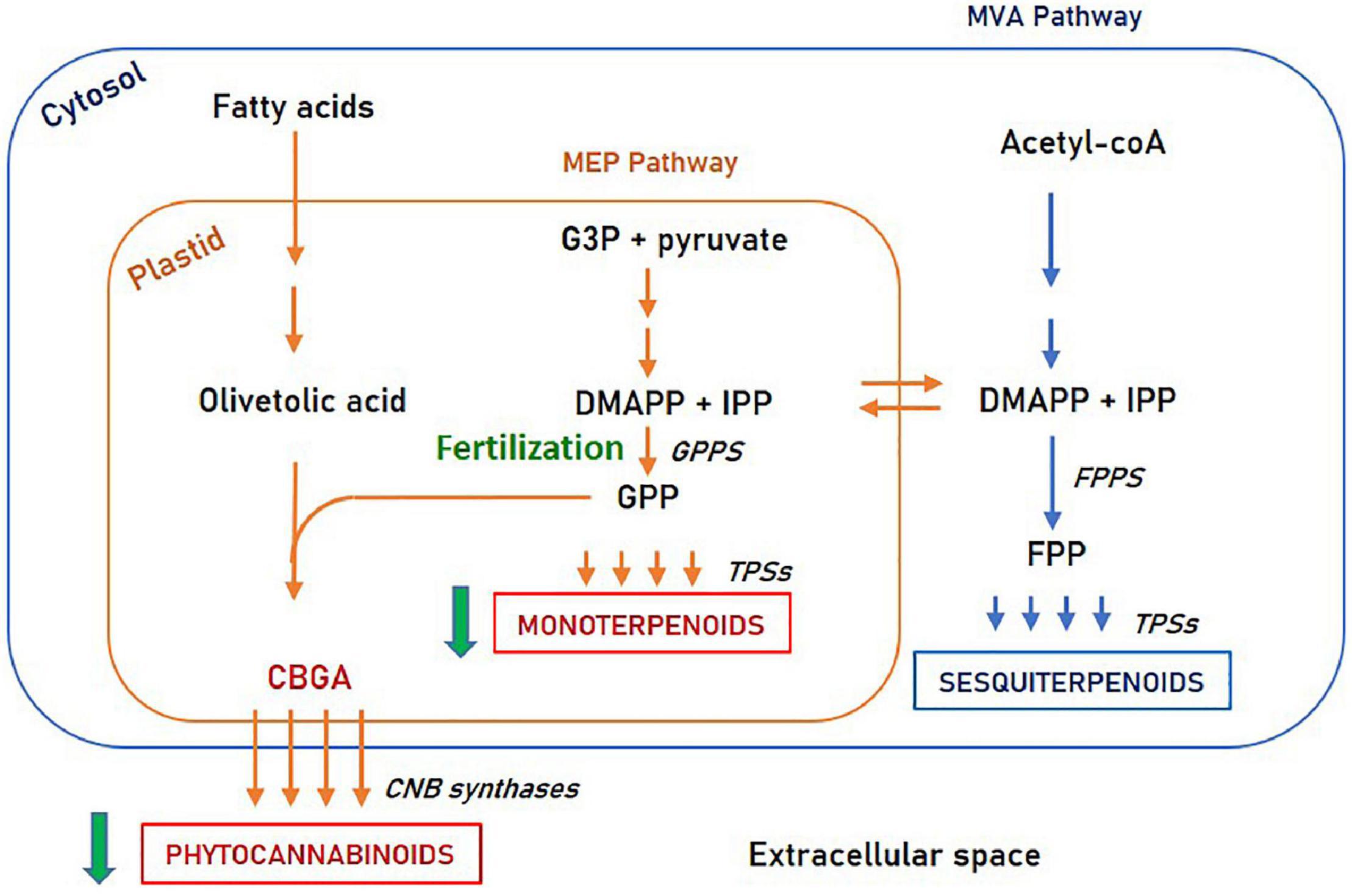
Second, this study revealed the resemblance between monoterpenoids and phytocannabinoids accumulation patterns. Both secondary metabolite species are decreased upon fertilization, while sesquiterpenoids are differently influenced. Possible explanations for this similarity are common intracellular regulation pathways or shared morphological structures. From a cellular perspective, monoterpenoids and phytocannabinoids share the common biosynthetic precursor geranyl diphosphate (GPP) and are both biosynthesized in the plastid.[14][35] In contrast, sesquiterpenoids are synthesized in the cytosol from a different precursor (farnesyl pyrophosphate or FPP). This suggests that phytocannabinoids and monoterpenoids may share a common regulation mechanism, involving an enzymatic step upstream to GPP, such as GPP synthase (illustrated in Figure 7).
|
Alternatively, from a morphological perspective, previous studies have shown that although phytocannabinoids, monoterpenoids, and sesquiterpenoids are all biosynthesized and accumulated in the glandular trichomes, their distribution differentiates during trichome development and between trichome types. A recent study by Booth et al.[14] showed an increase in the ratio of monoterpenoids relative to sesquiterpenoids when flowers are maturing. Another study by Livingston et al.[23] showed that monoterpenoids are accumulated in both pre-stalked and stalked trichomes, while sesquiterpenoids are abundant in sessile trichomes. Phytocannabinoids are accumulated in both types of trichomes, but the stalked type composed 80–90% of the total trichomes in the mature flower. A common accumulation pattern of monoterpenoids and phytocannabinoids during flower development was also previously demonstrated.[48] Parallel accumulation and decrease of phytocannabinoids and monoterpenoids in contrast to sesquiterpenoids may suggest that trichome types are differently affected by fertilization, and hence the diversity in metabolite accumulation.
An additional major finding depicted in this study is the somewhat dependent outcome of the fertilization process on the pollen donor plant. Both THC- or CBD-rich male plants, whether naturally occurring or female-induced, had a different impact on the metabolite concentration in the female after fertilization. For instance, fertilization by the induced-male led to an increase of specific phytocannabinoids (Figure 2): THC and THCA in the CBD female, and CBC, CBCA, CBCVA, and 373-15C in the THC female. The exact mechanism by which these phytocannabinoids are increased is not yet clear. It may be the result of altered regulation of synthesis enzymes, for example the upregulation of THCA synthase or CBCA synthase.[21] A previous study found over 10,000 genes are differentially expressed upon masculinization of female plants[49], but it is not clear how these genes are related to phytocannabinoid expression in the fertilized female plant. A donor-dependent effect was also detected in the specific increase in the eudesmol family components, which were highly increased in the THC-rich female upon fertilization by the THC-rich male plant (Figure 6A) and a parallel specific increase in linalool in the CBD-rich female fertilized by the induced male (Figure 6B). However, regardless of the type of male plant used for fertilization, the overall profile of the phytocannabinoids in the fertilized female plant remained unaltered, i.e., no new phytocannabinoids that were not expressed in the unfertilized plant were discovered, and the relative ratio between the different phytocannabinoids was mostly kept. Interestingly, though the density of phytocannabinoids and terpenoids in males is minor (data not shown) compared to the female flowers, with high potency female plants showing 10 to 20 times more THC than corresponding males[50], male plants also possess a distinct profile of these compounds.
Conclusion
Here, we used highly advanced analytical methods to thoroughly assess the composition of 95 phytocannabinoids and 113 terpenoids in the inflorescences of female plants fertilized by different males, including the female plant itself induced to develop male pollen sacs. We found that fertilization significantly decreased phytocannabinoids content, while terpenoids were differentially affected. To further elucidate the effect of fertilization on the secondary metabolite accumulation, future studies that follow the gene expression of enzymes upstream to GPP after fertilization may allow exposing master regulators of the biochemical pathways. In addition, better characterization of the morphological changes following fertilization may shed light on how different trichome types are affected by fertilization. Finally, the variance in metabolites observed by fertilization with different male plants may suggest that the pollen itself or the developing embryo influence the female sporophyte.
Altogether, one must remember that these specialized secondary metabolites have an important role in planta, increasing the plant fitness to the environment.[51] The substantial decrease in phytocannabinoids and terpenoids after fertilization may point to their functional roles in the plant. The actual functions of phytocannabinoids and terpenoids in Cannabis were only sparsely studied, mainly suggesting roles in defense against biotic or abiotic factors[40], protection from UV radiation[52], prevention of desiccation[12], or induction of cell death in leaves.[53] The observed dynamics of the accumulation of these metabolites during flower development and fertilization may point to their different roles along the plant’s life cycle.
Supplementary material
The supplementary material for this article can be found online at frontiersin.org.
Acknowledgements
We thank Dr. Ari Feder for helpful discussions and insights.
Author contributions
PB, MF, and DM: conception and design. OC, IK, and RP: plant maintenance, manipulations, fertilization, and harvest. CL: acquisition and ESI-LC/MS analysis and interpretation of data. AS: acquisition of GC data and development of semi-quantitative GC methodology. OG: extraction and sample preparation of Cannabis samples and UHPLC/UV analysis. CL, AS, and SP: figure preparation. CL, SP, PB, MF, and DM: writing, review, and revision of the manuscript. MF and DM: study supervision. All authors contributed to the article and approved the submitted version.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- ↑ Bonini, Sara Anna; Premoli, Marika; Tambaro, Simone; Kumar, Amit; Maccarinelli, Giuseppina; Memo, Maurizio; Mastinu, Andrea (1 December 2018). "Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history" (in en). Journal of Ethnopharmacology 227: 300–315. doi:10.1016/j.jep.2018.09.004. https://linkinghub.elsevier.com/retrieve/pii/S0378874118316611.
- ↑ Fernández-Ruiz, Javier (1 May 2019). "The biomedical challenge of neurodegenerative disorders: an opportunity for cannabinoid-based therapies to improve on the poor current therapeutic outcomes: Cannabinoids and neuroprotection" (in en). British Journal of Pharmacology 176 (10): 1370–1383. doi:10.1111/bph.14382. PMC PMC6487558. PMID 29856067. https://onlinelibrary.wiley.com/doi/10.1111/bph.14382.
- ↑ Cassano, Tommaso; Villani, Rosanna; Pace, Lorenzo; Carbone, Antonio; Bukke, Vidyasagar Naik; Orkisz, Stanislaw; Avolio, Carlo; Serviddio, Gaetano (6 March 2020). "From Cannabis sativa to Cannabidiol: Promising Therapeutic Candidate for the Treatment of Neurodegenerative Diseases". Frontiers in Pharmacology 11: 124. doi:10.3389/fphar.2020.00124. ISSN 1663-9812. PMC PMC7069528. PMID 32210795. https://www.frontiersin.org/article/10.3389/fphar.2020.00124/full.
- ↑ Starowicz, Katarzyna; Finn, David P. (2017), "Cannabinoids and Pain: Sites and Mechanisms of Action" (in en), Advances in Pharmacology (Elsevier) 80: 437–475, doi:10.1016/bs.apha.2017.05.003, ISBN 978-0-12-811232-8, https://linkinghub.elsevier.com/retrieve/pii/S1054358917300443. Retrieved 2021-11-24
- ↑ Franco, Valentina; Bialer, Meir; Perucca, Emilio (1 March 2021). "Cannabidiol in the treatment of epilepsy: Current evidence and perspectives for further research" (in en). Neuropharmacology 185: 108442. doi:10.1016/j.neuropharm.2020.108442. https://linkinghub.elsevier.com/retrieve/pii/S0028390820305104.
- ↑ Rice, Jessica; Cameron, Michelle (1 August 2018). "Cannabinoids for Treatment of MS Symptoms: State of the Evidence" (in en). Current Neurology and Neuroscience Reports 18 (8): 50. doi:10.1007/s11910-018-0859-x. ISSN 1528-4042. http://link.springer.com/10.1007/s11910-018-0859-x.
- ↑ Gonçalves, Joana; Rosado, Tiago; Soares, Sofia; Simão, Ana; Caramelo, Débora; Luís, Ângelo; Fernández, Nicolás; Barroso, Mário et al. (23 February 2019). "Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination" (in en). Medicines 6 (1): 31. doi:10.3390/medicines6010031. ISSN 2305-6320. PMC PMC6473697. PMID 30813390. http://www.mdpi.com/2305-6320/6/1/31.
- ↑ Andre, Christelle M.; Hausman, Jean-Francois; Guerriero, Gea (4 February 2016). "Cannabis sativa: The Plant of the Thousand and One Molecules". Frontiers in Plant Science 7: 19. doi:10.3389/fpls.2016.00019. ISSN 1664-462X. PMC PMC4740396. PMID 26870049. http://journal.frontiersin.org/Article/10.3389/fpls.2016.00019/abstract.
- ↑ ElSohly, Mahmoud A.; Slade, Desmond (1 December 2005). "Chemical constituents of marijuana: The complex mixture of natural cannabinoids" (in en). Life Sciences 78 (5): 539–548. doi:10.1016/j.lfs.2005.09.011. https://linkinghub.elsevier.com/retrieve/pii/S002432050500891X.
- ↑ 10.0 10.1 10.2 Flores-Sanchez, Isvett Josefina; Verpoorte, Robert (1 October 2008). "Secondary metabolism in cannabis" (in en). Phytochemistry Reviews 7 (3): 615–639. doi:10.1007/s11101-008-9094-4. ISSN 1568-7767. http://link.springer.com/10.1007/s11101-008-9094-4.
- ↑ 11.0 11.1 Hanuš, Lumír Ondřej; Meyer, Stefan Martin; Muñoz, Eduardo; Taglialatela-Scafati, Orazio; Appendino, Giovanni (2016). "Phytocannabinoids: a unified critical inventory" (in en). Natural Product Reports 33 (12): 1357–1392. doi:10.1039/C6NP00074F. ISSN 0265-0568. http://xlink.rsc.org/?DOI=C6NP00074F.
- ↑ 12.0 12.1 Gülck, Thies; Møller, Birger Lindberg (1 October 2020). "Phytocannabinoids: Origins and Biosynthesis" (in en). Trends in Plant Science 25 (10): 985–1004. doi:10.1016/j.tplants.2020.05.005. https://linkinghub.elsevier.com/retrieve/pii/S1360138520301874.
- ↑ 13.0 13.1 13.2 13.3 13.4 Berman, Paula; Futoran, Kate; Lewitus, Gil M.; Mukha, Dzmitry; Benami, Maya; Shlomi, Tomer; Meiri, David (1 December 2018). "A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis" (in en). Scientific Reports 8 (1): 14280. doi:10.1038/s41598-018-32651-4. ISSN 2045-2322. PMC PMC6155167. PMID 30250104. http://www.nature.com/articles/s41598-018-32651-4.
- ↑ 14.0 14.1 14.2 14.3 14.4 Booth, Judith K.; Yuen, Macaire M.S.; Jancsik, Sharon; Madilao, Lufiani L.; Page, Jonathan E.; Bohlmann, Jörg (1 September 2020). "Terpene Synthases and Terpene Variation in Cannabis sativa" (in en). Plant Physiology 184 (1): 130–147. doi:10.1104/pp.20.00593. ISSN 0032-0889. PMC PMC7479917. PMID 32591428. https://academic.oup.com/plphys/article/184/1/130-147/6117797.
- ↑ 15.0 15.1 Russo, Ethan B.; Marcu, Jahan (2017), "Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads" (in en), Advances in Pharmacology (Elsevier) 80: 67–134, doi:10.1016/bs.apha.2017.03.004, ISBN 978-0-12-811232-8, https://linkinghub.elsevier.com/retrieve/pii/S1054358917300273. Retrieved 2021-11-24
- ↑ Radwan, Mohamed M.; ElSohly, Mahmoud A.; Slade, Desmond; Ahmed, Safwat A.; Wilson, Lisa; El-Alfy, Abir T.; Khan, Ikhlas A.; Ross, Samir A. (1 October 2008). "Non-cannabinoid constituents from a high potency Cannabis sativa variety" (in en). Phytochemistry 69 (14): 2627–2633. doi:10.1016/j.phytochem.2008.07.010. PMC PMC4888767. PMID 18774146. https://linkinghub.elsevier.com/retrieve/pii/S0031942208003518.
- ↑ Rea, Kevin A; Casaretto, José A.; Al-Abdul-Wahid, M. Sameer; Sukumaran, Arjun; Geddes-McAlister, Jennifer; Rothstein, Steven J.; Akhtar, Tariq A. (1 August 2019). "Biosynthesis of cannflavins A and B from Cannabis sativa L" (in en). Phytochemistry 164: 162–171. doi:10.1016/j.phytochem.2019.05.009. https://linkinghub.elsevier.com/retrieve/pii/S0031942218303819.
- ↑ Erridge, Simon; Mangal, Nagina; Salazar, Oliver; Pacchetti, Barbara; Sodergren, Mikael H. (1 October 2020). "Cannflavins – From plant to patient: A scoping review" (in en). Fitoterapia 146: 104712. doi:10.1016/j.fitote.2020.104712. https://linkinghub.elsevier.com/retrieve/pii/S0367326X2030294X.
- ↑ 19.0 19.1 19.2 19.3 Shapira, Anna; Berman, Paula; Futoran, Kate; Guberman, Ohad; Meiri, David (3 September 2019). "Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections" (in en). Analytical Chemistry 91 (17): 11425–11432. doi:10.1021/acs.analchem.9b02844. ISSN 0003-2700. https://pubs.acs.org/doi/10.1021/acs.analchem.9b02844.
- ↑ van Bakel, Harm; Stout, Jake M; Cote, Atina G; Tallon, Carling M; Sharpe, Andrew G; Hughes, Timothy R; Page, Jonathan E (2011). "The draft genome and transcriptome of Cannabis sativa" (in en). Genome Biology 12 (10): R102. doi:10.1186/gb-2011-12-10-r102. ISSN 1465-6906. PMC PMC3359589. PMID 22014239. http://genomebiology.biomedcentral.com/articles/10.1186/gb-2011-12-10-r102.
- ↑ 21.0 21.1 Laverty, Kaitlin U.; Stout, Jake M.; Sullivan, Mitchell J.; Shah, Hardik; Gill, Navdeep; Holbrook, Larry; Deikus, Gintaras; Sebra, Robert et al. (1 January 2019). "A physical and genetic map of Cannabis sativa identifies extensive rearrangements at the THC/CBD acid synthase loci" (in en). Genome Research 29 (1): 146–156. doi:10.1101/gr.242594.118. ISSN 1088-9051. PMC PMC6314170. PMID 30409771. http://genome.cshlp.org/lookup/doi/10.1101/gr.242594.118.
- ↑ Vincent, Delphine; Rochfort, Simone; Spangenberg, German (13 February 2019). "Optimisation of Protein Extraction from Medicinal Cannabis Mature Buds for Bottom-Up Proteomics" (in en). Molecules 24 (4): 659. doi:10.3390/molecules24040659. ISSN 1420-3049. PMC PMC6412734. PMID 30781766. http://www.mdpi.com/1420-3049/24/4/659.
- ↑ 23.0 23.1 23.2 Livingston, Samuel J.; Quilichini, Teagen D.; Booth, Judith K.; Wong, Darren C. J.; Rensing, Kim H.; Laflamme‐Yonkman, Jessica; Castellarin, Simone D.; Bohlmann, Joerg et al. (1 January 2020). "Cannabis glandular trichomes alter morphology and metabolite content during flower maturation" (in en). The Plant Journal 101 (1): 37–56. doi:10.1111/tpj.14516. ISSN 0960-7412. https://onlinelibrary.wiley.com/doi/10.1111/tpj.14516.
- ↑ McGarvey, Peter; Huang, Jiahao; McCoy, Matthew; Orvis, Joshua; Katsir, Yael; Lotringer, Nitzan; Nesher, Iris; Kavarana, Malcolm et al. (1 December 2020). "De novo assembly and annotation of transcriptomes from two cultivars of Cannabis sativa with different cannabinoid profiles" (in en). Gene 762: 145026. doi:10.1016/j.gene.2020.145026. https://linkinghub.elsevier.com/retrieve/pii/S0378111920306958.
- ↑ Cai, Sen; Zhang, Zhiyuan; Huang, Suyun; Bai, Xu; Huang, Ziying; Zhang, Yiping Jason; Huang, Likun; Tang, Weiqi et al. (1 May 2021). "CannabisGDB: a comprehensive genomic database for Cannabis Sativa L" (in en). Plant Biotechnology Journal 19 (5): 857–859. doi:10.1111/pbi.13548. ISSN 1467-7644. PMC PMC8131054. PMID 33462958. https://onlinelibrary.wiley.com/doi/10.1111/pbi.13548.
- ↑ Booth, Judith K.; Page, Jonathan E.; Bohlmann, Jörg (29 March 2017). Hamberger, Björn. ed. "Terpene synthases from Cannabis sativa" (in en). PLOS ONE 12 (3): e0173911. doi:10.1371/journal.pone.0173911. ISSN 1932-6203. PMC PMC5371325. PMID 28355238. https://dx.plos.org/10.1371/journal.pone.0173911.
- ↑ Allen, Keith D.; McKernan, Kevin; Pauli, Christopher; Roe, Jim; Torres, Anthony; Gaudino, Reggie (12 September 2019). Hamberger, Björn. ed. "Genomic characterization of the complete terpene synthase gene family from Cannabis sativa" (in en). PLOS ONE 14 (9): e0222363. doi:10.1371/journal.pone.0222363. ISSN 1932-6203. PMC PMC6742361. PMID 31513654. https://dx.plos.org/10.1371/journal.pone.0222363.
- ↑ Zager, Jordan J.; Lange, Iris; Srividya, Narayanan; Smith, Anthony; Lange, B. Markus (1 August 2019). "Gene Networks Underlying Cannabinoid and Terpenoid Accumulation in Cannabis" (in en). Plant Physiology 180 (4): 1877–1897. doi:10.1104/pp.18.01506. ISSN 0032-0889. PMC PMC6670104. PMID 31138625. https://academic.oup.com/plphys/article/180/4/1877-1897/6117720.
- ↑ Hawley, Dave; Graham, Thomas; Stasiak, Michael; Dixon, Mike (1 November 2018). "Improving Cannabis Bud Quality and Yield with Subcanopy Lighting". HortScience 53 (11): 1593–1599. doi:10.21273/HORTSCI13173-18. ISSN 0018-5345. https://journals.ashs.org/view/journals/hortsci/53/11/article-p1593.xml.
- ↑ Magagnini, Gianmaria; Grassi, Gianpaolo; Kotiranta, Stiina (12 June 2018). "The Effect of Light Spectrum on the Morphology and Cannabinoid Content of Cannabis sativa L." (in en). Medical Cannabis and Cannabinoids 1 (1): 19–27. doi:10.1159/000489030. ISSN 2504-3889. PMC PMC8489345. PMID 34676318. https://www.karger.com/Article/FullText/489030.
- ↑ Namdar, Dvory; Charuvi, Dana; Ajjampura, Vinayka; Mazuz, Moran; Ion, Aurel; Kamara, Itzhak; Koltai, Hinanit (1 June 2019). "LED lighting affects the composition and biological activity of Cannabis sativa secondary metabolites" (in en). Industrial Crops and Products 132: 177–185. doi:10.1016/j.indcrop.2019.02.016. https://linkinghub.elsevier.com/retrieve/pii/S0926669019301086.
- ↑ 32.0 32.1 Meier, C.; Mediavilla, V. (1998). "Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil". Journal of the International Hemp Association 5 (1): 16–20. http://www.internationalhempassociation.org/jiha/jiha5107.html.
- ↑ Bernstein, Nirit; Gorelick, Jonathan; Zerahia, Roei; Koch, Sraya (17 June 2019). "Impact of N, P, K, and Humic Acid Supplementation on the Chemical Profile of Medical Cannabis (Cannabis sativa L)". Frontiers in Plant Science 10: 736. doi:10.3389/fpls.2019.00736. ISSN 1664-462X. PMC PMC6589925. PMID 31263470. https://www.frontiersin.org/article/10.3389/fpls.2019.00736/full.
- ↑ Chandra, Suman; Lata, Hemant; ElSohly, Mahmoud A. (26 June 2020). "Propagation of Cannabis for Clinical Research: An Approach Towards a Modern Herbal Medicinal Products Development". Frontiers in Plant Science 11: 958. doi:10.3389/fpls.2020.00958. ISSN 1664-462X. PMC PMC7333344. PMID 32676092. https://www.frontiersin.org/article/10.3389/fpls.2020.00958/full.
- ↑ 35.0 35.1 Romero, P.; Peris, A.; Vergara, K.; Matus, J.T. (1 September 2020). "Comprehending and improving cannabis specialized metabolism in the systems biology era" (in en). Plant Science 298: 110571. doi:10.1016/j.plantsci.2020.110571. https://linkinghub.elsevier.com/retrieve/pii/S0168945220301771.
- ↑ O'Neill, Sharman D. (1 June 1997). "POLLINATION REGULATION OF FLOWER DEVELOPMENT" (in en). Annual Review of Plant Physiology and Plant Molecular Biology 48 (1): 547–574. doi:10.1146/annurev.arplant.48.1.547. ISSN 1040-2519. https://www.annualreviews.org/doi/10.1146/annurev.arplant.48.1.547.
- ↑ Tripathi, Siddharth Kaushal; Tuteja, Narendra (1 November 2007). "Integrated Signaling in Flower Senescence: An Overview" (in en). Plant Signaling & Behavior 2 (6): 437–445. doi:10.4161/psb.2.6.4991. ISSN 1559-2324. PMC PMC2634333. PMID 19517004. http://www.tandfonline.com/doi/abs/10.4161/psb.2.6.4991.
- ↑ Borghi, Monica; Fernie, Alisdair R. (1 August 2020). "Outstanding questions in flower metabolism" (in en). The Plant Journal 103 (4): 1275–1288. doi:10.1111/tpj.14814. ISSN 0960-7412. https://onlinelibrary.wiley.com/doi/10.1111/tpj.14814.
- ↑ Potter, David J.; Hammond, Kathy; Tuffnell, Shaun; Walker, Christopher; Di Forti, Marta (1 April 2018). "Potency of Δ 9 -tetrahydrocannabinol and other cannabinoids in cannabis in England in 2016: Implications for public health and pharmacology" (in en). Drug Testing and Analysis 10 (4): 628–635. doi:10.1002/dta.2368. https://onlinelibrary.wiley.com/doi/10.1002/dta.2368.
- ↑ 40.0 40.1 Potter, D. (March 2009). "The Propagation, Characterisation and Optimisation of Cannabis sativa L. as a Phytopharmaceutical" (PDF). King’s College London. https://extractionmagazine.com/wp-content/uploads/2018/06/THE-PROPAGATION-CHARACTERISATION-AND-OPTIMISATION-OF-CANNABIS-SATIVA-L-AS-A-PHYTOPHARMACEUTICAL.pdf.
- ↑ Mohan Ram, H. Y.; Sett, R. (1 December 1982). "Induction of fertile male flowers in genetically female Cannabis sativa plants by silver nitrate and silver thiosulphate anionic complex" (in en). Theoretical and Applied Genetics 62 (4): 369–375. doi:10.1007/BF00275107. ISSN 0040-5752. http://link.springer.com/10.1007/BF00275107.
- ↑ Small, Ernest; Naraine, Steve G. U. (1 February 2016). "Expansion of female sex organs in response to prolonged virginity in Cannabis sativa (marijuana)" (in en). Genetic Resources and Crop Evolution 63 (2): 339–348. doi:10.1007/s10722-015-0253-3. ISSN 0925-9864. http://link.springer.com/10.1007/s10722-015-0253-3.
- ↑ Milay, Looz; Berman, Paula; Shapira, Anna; Guberman, Ohad; Meiri, David (15 October 2020). "Metabolic Profiling of Cannabis Secondary Metabolites for Evaluation of Optimal Postharvest Storage Conditions". Frontiers in Plant Science 11: 583605. doi:10.3389/fpls.2020.583605. ISSN 1664-462X. PMC PMC7593247. PMID 33178249. https://www.frontiersin.org/article/10.3389/fpls.2020.583605/full.
- ↑ Livingston, Samuel J.; Quilichini, Teagen D.; Booth, Judith K.; Wong, Darren C. J.; Rensing, Kim H.; Laflamme‐Yonkman, Jessica; Castellarin, Simone D.; Bohlmann, Joerg et al. (1 January 2020). "Cannabis glandular trichomes alter morphology and metabolite content during flower maturation" (in en). The Plant Journal 101 (1): 37–56. doi:10.1111/tpj.14516. ISSN 0960-7412. https://onlinelibrary.wiley.com/doi/10.1111/tpj.14516.
- ↑ Huang, Jian; Smith, Ashley R.; Zhang, Tianyu; Zhao, Dazhong (1 February 2016). "Creating Completely Both Male and Female Sterile Plants by Specifically Ablating Microspore and Megaspore Mother Cells". Frontiers in Plant Science 7. doi:10.3389/fpls.2016.00030. ISSN 1664-462X. PMC PMC4740954. PMID 26870055. http://journal.frontiersin.org/article/10.3389/fpls.2016.00030.
- ↑ Jung, Yu Jin; Kim, Dong Hyun; Lee, Hyo Ju; Nam, Ki Hong; Bae, Sangsu; Nou, Ill Sup; Cho, Yong-Gu; Kim, Myong Kwon et al. (11 September 2020). "Knockout of SlMS10 Gene (Solyc02g079810) Encoding bHLH Transcription Factor Using CRISPR/Cas9 System Confers Male Sterility Phenotype in Tomato" (in en). Plants 9 (9): 1189. doi:10.3390/plants9091189. ISSN 2223-7747. PMC PMC7570381. PMID 32933074. https://www.mdpi.com/2223-7747/9/9/1189.
- ↑ Zhu, Haocheng; Li, Chao; Gao, Caixia (1 November 2020). "Applications of CRISPR–Cas in agriculture and plant biotechnology" (in en). Nature Reviews Molecular Cell Biology 21 (11): 661–677. doi:10.1038/s41580-020-00288-9. ISSN 1471-0072. https://www.nature.com/articles/s41580-020-00288-9.
- ↑ Aizpurua-Olaizola, Oier; Soydaner, Umut; Öztürk, Ekin; Schibano, Daniele; Simsir, Yilmaz; Navarro, Patricia; Etxebarria, Nestor; Usobiaga, Aresatz (26 February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes" (in en). Journal of Natural Products 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. ISSN 0163-3864. https://pubs.acs.org/doi/10.1021/acs.jnatprod.5b00949.
- ↑ Adal, Ayelign M.; Doshi, Ketan; Holbrook, Larry; Mahmoud, Soheil S. (1 January 2021). "Comparative RNA-Seq analysis reveals genes associated with masculinization in female Cannabis sativa" (in en). Planta 253 (1): 17. doi:10.1007/s00425-020-03522-y. ISSN 0032-0935. PMC PMC7779414. PMID 33392743. http://link.springer.com/10.1007/s00425-020-03522-y.
- ↑ Clarke, R.; Merlin, M. (2016). Cannabis: Evolution and Ethnobotany (First ed.). University of California Press. pp. 456. ISBN 9780520292482. https://www.ucpress.edu/book/9780520292482/cannabis.
- ↑ Huchelmann, Alexandre; Boutry, Marc; Hachez, Charles (1 September 2017). "Plant Glandular Trichomes: Natural Cell Factories of High Biotechnological Interest" (in en). Plant Physiology 175 (1): 6–22. doi:10.1104/pp.17.00727. ISSN 0032-0889. PMC PMC5580781. PMID 28724619. https://academic.oup.com/plphys/article/175/1/6-22/6117034.
- ↑ Eichhorn Bilodeau, Samuel; Wu, Bo-Sen; Rufyikiri, Anne-Sophie; MacPherson, Sarah; Lefsrud, Mark (29 March 2019). "An Update on Plant Photobiology and Implications for Cannabis Production". Frontiers in Plant Science 10: 296. doi:10.3389/fpls.2019.00296. ISSN 1664-462X. PMC PMC6455078. PMID 31001288. https://www.frontiersin.org/article/10.3389/fpls.2019.00296/full.
- ↑ Morimoto, Satoshi; Tanaka, Yumi; Sasaki, Kaori; Tanaka, Hiroyuki; Fukamizu, Tomohide; Shoyama, Yoshinari; Shoyama, Yukihiro; Taura, Futoshi (1 July 2007). "Identification and Characterization of Cannabinoids That Induce Cell Death through Mitochondrial Permeability Transition in Cannabis Leaf Cells" (in en). Journal of Biological Chemistry 282 (28): 20739–20751. doi:10.1074/jbc.M700133200. https://linkinghub.elsevier.com/retrieve/pii/S0021925819780661.
Notes
This presentation is faithful to the original, with only a few minor changes to presentation. Some grammar and punctuation was cleaned up to improve readability. In some cases important information was missing from the references, and that information was added. The original article lists references in alphabetical order; however, this version lists them in order of appearance, by design.